Abstract
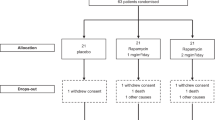
Amyotrophic lateral sclerosis (ALS) is characterized by upper and lower motor neuron dysfunction and loss, rapidly progressive muscle weakness, wasting and death1,2,3. Many factors, including mitochondrial dysfunction, may contribute to ALS pathogenesis4,5,6,7,8,9. Riluzole, which has shown only modest benefits in a measure of survival time without demonstrated effects on muscle strength or function, is the only approved treatment for ALS10,11. We tested the putative mitochondrial modulator dexpramipexole (KNS-760704; (6R)-4,5,6,7-tetrahydro-N6-propyl-2,6-benzothiazole-diamine)12,13,14 in subjects with ALS in a two-part, double-blind safety and tolerability study, with a preliminary assessment of its effects on functional decline and mortality. In part 1, the effects of dexpramipexole (50, 150 or 300 mg d−1) versus placebo were assessed over 12 weeks. In part 2, after a 4-week, single-blind placebo washout, continuing subjects were re-randomized to dexpramipexole at 50 mg d−1 or 300 mg d−1 as double-blind active treatment for 24 weeks. Dexpramipexole was safe and well tolerated. Trends showing a dose-dependent attenuation of the slope of decline of the ALS Functional Rating Scale-Revised (ALSFRS-R) in part 1 and a statistically significant (P = 0.046) difference between groups in a joint rank test of change from baseline in ALSFRS-R and mortality in part 2 strongly support further testing of dexpramipexole in ALS.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Rowland, L.P. & Shneider, N.A. Amyotrophic lateral sclerosis. N. Engl. J. Med. 344, 1688–1700 (2001).
Wijesekera, L.C. & Leigh, P.N. Amyotrophic lateral sclerosis. Orphanet J. Rare Dis. 4, 3 (2009).
Vucic, S. & Kiernan, M.C. Pathophysiology of neurodegeneration in familial amyotrophic lateral sclerosis. Curr. Mol. Med. 9, 255–272 (2009).
Hervias, I., Beal, M.F. & Manfredi, G. Mitochondrial dysfunction and amyotrophic lateral sclerosis. Muscle Nerve 33, 598–608 (2006).
Manfredi, G. & Xu, Z. Mitochondrial dysfunction and its role in motor neuron degeneration in ALS. Mitochondrion 5, 77–87 (2005).
Baron, M., Kudin, A.P. & Kunz, W.S. Mitochondrial dysfunction in neurodegenerative disorders. Biochem. Soc. Trans. 35, 1228–1231 (2007).
Corti, S. et al. Amyotrophic lateral sclerosis linked to a novel SOD1 mutation with muscle mitochondrial dysfunction. J. Neurol. Sci. 276, 170–174 (2009).
Kawamata, H. & Manfredi, G. Mitochondrial dysfunction and intracellular calcium dysregulation in ALS. Mech. Ageing Dev. 131, 517–526 (2010).
Shi, P., Wei, Y., Zhang, J., Gal, J. & Zhu, H. Mitochondrial dysfunction is a converging point of multiple pathological pathways in amyotrophic lateral sclerosis. J. Alzheimers Dis. 20 (suppl. 2), S311–S324 (2010).
Messori, A., Trippoli, S., Becagli, P. & Zaccara, G. Cost effectiveness of riluzole in amyotrophic lateral sclerosis. Italian Cooperative Group for the Study of Meta-Analysis and the Osservatorio SIFO sui Farmaci. Pharmacoeconomics 16, 153–163 (1999).
Stewart, A. et al. The clinical effectiveness and cost-effectiveness of riluzole for motor neurone disease: a rapid and systematic review. Health Technol. Assess. 5, 1–97 (2001).
Gribkoff, V.K. & Bozik, M.E. KNS-760704 [(6R)-4,5,6,7-tetrahydro-N6-propyl-2, 6-benzothiazole-diamine dihydrochloride monohydrate] for the treatment of amyotrophic lateral sclerosis. CNS Neurosci. Ther. 14, 215–226 (2008).
Wang, H. et al. R+ pramipexole as a mitochondrially focused neuroprotectant: initial early phase studies in ALS. Amyotroph. Lateral Scler. 9, 50–58 (2008).
Bozik, M.E., Mather, J.M., Kramer, W.H., Gribkoff, V.K. & Ingersoll, E.W. Safety, tolerability and pharmacokinetics of KNS-760704 (dexpramipexole) in healthy adult subjects. J. Clin. Pharmacol. 51, 1177–1185 (2010).
Cudkowicz, M.E. et al. Toward more efficient clinical trials for amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. 11, 259–265 (2010).
Cheung, Y.K., Gordon, P.H. & Levin, B. Selecting promising ALS therapies in clinical trials. Neurology 67, 1748–1751 (2006).
Groeneveld, G.J., Graf, M., van, d.T.I., van den Berg, L.H. & Ludolph, A.C. Alternative trial design in amyotrophic lateral sclerosis saves time and patients. Amyotroph. Lateral Scler. 8, 266–269 (2007).
Gordon, P.H. et al. Outcome measures for early phase clinical trials. Amyotroph. Lateral Scler. 8, 270–273 (2007).
Schoenfeld, D.A. & Cudkowicz, M. Design of phase II ALS clinical trials. Amyotroph. Lateral Scler. 9, 16–23 (2008).
Shefner, J.M. Designing clinical trials in amyotrophic lateral sclerosis. Phys. Med. Rehabil. Clin. N. Am. 19, 495–508 (2008).
Bedlack, R.S., Pastula, D.M., Welsh, E., Pulley, D. & Cudkowicz, M.E. Scrutinizing enrollment in ALS clinical trials: room for improvement? Amyotroph. Lateral Scler. 9, 257–265 (2008).
Laird, N.M. & Ware, J.H. Random-effects models for longitudinal data. Biometrics 38, 963–974 (1982).
Aggarwal, S.P. et al. Safety and efficacy of lithium in combination with riluzole for treatment of amyotrophic lateral sclerosis: a randomised, double-blind, placebo-controlled trial. Lancet Neurol. 9, 481–488 (2010).
Castrillo-Viguera, C., Grasso, D.L., Simpson, E., Shefner, J. & Cudkowicz, M.E. Clinical significance in the change of decline in ALSFRS-R. Amyotroph. Lateral Scler. 11, 178–180 (2010).
Cedarbaum, J.M. et al. The ALSFRS-R: a revised ALS functional rating scale that incorporates assessments of respiratory function. BDNF ALS Study Group (phase III). J. Neurol. Sci. 169, 13–21 (1999).
Miller, R. et al. Phase II/III randomized trial of TCH346 in patients with ALS. Neurology 69, 776–784 (2007).
Gordon, P.H. & Cheung, Y.K. Progression rate of ALSFRS-R at time of diagnosis predicts survival time in ALS. Neurology 67, 1314–1315 (2006).
Kollewe, K. et al. ALSFRS-R score and its ratio: a useful predictor for ALS-progression. J. Neurol. Sci. 275, 69–73 (2008).
Liu, X.X., Fan, D.S., Zhang, J., Zhang, S. & Zheng, J.Y. Zhonghua Yi Xue Za Zhi [Revised amyotrophic lateral sclerosis functional rating scale at time of diagnosis predicts survival time in amyotrophic lateral sclerosis] 89, 2472–2475 (2009).
Kaufmann, P. et al. The ALSFRSr predicts survival time in an ALS clinic population. Neurology 64, 38–43 (2005).
Gordon, P.H., Miller, R.G. & Moore, D.H. ALSFRS-R. Amyotroph. Lateral Scler. Other Motor Neuron Disord. 5 (suppl. 1), 90–93 (2004).
Kaufmann, P. et al. Excellent inter-rater, intra-rater and telephone-administered reliability of the ALSFRS-R in a multicenter clinical trial. Amyotroph. Lateral Scler. 8, 42–46 (2007).
Aggarwal, S.P. et al. Safety and efficacy of lithium in combination with riluzole for treatment of amyotrophic lateral sclerosis: a randomised, double-blind, placebo-controlled trial. Lancet Neurol. 9, 481–488 (2010).
Cohen, S.R. et al. Validity of the McGill Quality of Life Questionnaire in the palliative care setting: a multi-centre Canadian study demonstrating the importance of the existential domain. Palliat. Med. 11, 3–20 (1997).
Simmons, Z., Bremer, B.A., Robbins, R.A., Walsh, S.M. & Fischer, S. Quality of life in ALS depends on factors other than strength and physical function. Neurology 55, 388–392 (2000).
Gordon, P.H. et al. Efficacy of minocycline in patients with amyotrophic lateral sclerosis: a phase III randomised trial. Lancet Neurol. 6, 1045–1053 (2007).
Miller, R. et al. Phase II/III randomized trial of TCH346 in patients with ALS. Neurology 69, 776–784 (2007).
Cudkowicz, M.E. et al. Trial of celecoxib in amyotrophic lateral sclerosis. Ann. Neurol. 60, 22–31 (2006).
Finkelstein, D.M. & Schoenfeld, D.A. Combining mortality and longitudinal measures in clinical trials. Stat. Med. 18, 1341–1354 (1999).
Acknowledgements
We wish to thank the members of the KNS-760704-CL201 study group and acknowledge their substantial contributions to the success of this project (see list of members in the Supplementary Acknowledgments).
Author information
Authors and Affiliations
Contributions
M.C., R.M., H.M., J.S., D.H.M. and D.S. served as expert clinical and statistical advisors to the sponsor, Knopp Biosciences, and collaborated with M.E.B., E.W.I., J.L.M., D.A., M.S., C.A., J.M. and V.K.G. with respect to the design of the study. E.W.I., J.L.M., C.A., and J.M. were principally responsible for the clinical operations and execution of the study. M.E.B. was principally responsible for medical monitoring and patient safety assessments for the study. D.A. was principally responsible for the statistical analysis of the study with input from D.H.M. and D.S. who served in an advisory capacity. M.S. and E.W.I. were principally responsible for ensuring regulatory compliance with US federal regulations governing sponsored clinical research for the duration of the study. V.K.G., E.W.I., M.E.B., D.A., J.L.M. and M.C. collaborated to write the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1 and 2, Supplementary Tables 1–9 and Supplementary Methods (PDF 493 kb)
Rights and permissions
About this article
Cite this article
Cudkowicz, M., Bozik, M., Ingersoll, E. et al. The effects of dexpramipexole (KNS-760704) in individuals with amyotrophic lateral sclerosis. Nat Med 17, 1652–1656 (2011). https://doi.org/10.1038/nm.2579
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.2579
This article is cited by
-
AZD5438 a GSK-3a/b and CDK inhibitor is antiapoptotic modulates mitochondrial activity and protects human neurons from mitochondrial toxins
Scientific Reports (2023)
-
Neurofilament light and heterogeneity of disease progression in amyotrophic lateral sclerosis: development and validation of a prediction model to improve interventional trials
Translational Neurodegeneration (2021)
-
Dexpramipexole Enhances K+ Currents and Inhibits Cell Excitability in the Rat Hippocampus In Vitro
Molecular Neurobiology (2021)
-
Parkinson’s disease protein DJ-1 regulates ATP synthase protein components to increase neuronal process outgrowth
Cell Death & Disease (2019)
-
Cerebrospinal Fluid from Patients with Sporadic Amyotrophic Lateral Sclerosis Induces Degeneration of Motor Neurons Derived from Human Embryonic Stem Cells
Molecular Neurobiology (2019)