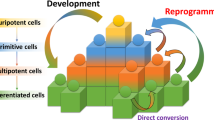
Abstract
The derivation of induced pluripotent cells (iPSCs) from individuals suffering from genetic syndromes offers new opportunities for basic research into these diseases and the development of therapeutic compounds. iPSCs can self renew and can be differentiated to many cell types, offering a potentially unlimited source of material for study. In this review we discuss the conceptual and practical issues to consider when attempting to model genetic diseases using iPSCs.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Odom, D.T. et al. Tissue-specific transcriptional regulation has diverged significantly between human and mouse. Nat. Genet. 39, 730–732 (2007).
Wilson, J.M. Animal models of human disease for gene therapy. J. Clin. Invest. 97, 1138–1141 (1996).
Perel, P. et al. Comparison of treatment effects between animal experiments and clinical trials: systematic review. Br. Med. J. 334, 197 (2007).
Savitz, S.I. A critical appraisal of the NXY-059 neuroprotection studies for acute stroke: a need for more rigorous testing of neuroprotective agents in animal models of stroke. Exp. Neurol. 205, 20–25 (2007).
Gearhart, J. New potential for human embryonic stem cells. Science 282, 1061–1062 (1998).
Thomson, J.A. et al. Embryonic stem cell lines derived from human blastocysts. Science 282, 1145–1147 (1998).
Mateizel, I. et al. Derivation of human embryonic stem cell lines from embryos obtained after IVF and after PGD for monogenic disorders. Hum. Reprod. 21, 503–511 (2006).
Sermon, K.D. et al. Creation of a registry for human embryonic stem cells carrying an inherited defect: joint collaboration between ESHRE and hESCreg. Hum. Reprod. 24, 1556–1560 (2009).
Urbach, A., Schuldiner, M. & Benvenisty, N. Modeling for Lesch-Nyhan disease by gene targeting in human embryonic stem cells. Stem Cells 22, 635–641 (2004).
Giudice, A. & Trounson, A. Genetic modification of human embryonic stem cells for derivation of target cells. Cell Stem Cell 2, 422–433 (2008).
Takahashi, K. & Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126, 663–676 (2006).
Dimos, J.T. et al. Induced pluripotent stem cells generated from patients with ALS can be differentiated into motor neurons. Science 321, 1218–1221 (2008).
Ebert, A.D. et al. Induced pluripotent stem cells from a spinal muscular atrophy patient. Nature 457, 277–280 (2009).
Lee, G. et al. Modelling pathogenesis and treatment of familial dysautonomia using patient-specific iPSCs. Nature 461, 402–406 (2009).
Marchetto, M.C. et al. A model for neural development and treatment of Rett syndrome using human induced pluripotent stem cells. Cell 143, 527–539 (2010).
Itzhaki, I. et al. Modelling the long QT syndrome with induced pluripotent stem cells. Nature 471, 225–229 (2011).
Brennand, K.J. et al. Modelling schizophrenia using human induced pluripotent stem cells. Nature 473, 221–225 (2011).
Liu, G.H. et al. Recapitulation of premature ageing with iPSCs from Hutchinson-Gilford progeria syndrome. Nature 472, 221–225 (2011).
Raya, A. et al. Disease-corrected haematopoietic progenitors from Fanconi anaemia induced pluripotent stem cells. Nature 460, 53–59 (2009).
González, F., Boue, S. & Izpisua Belmonte, J.C. Methods for making induced pluripotent stem cells: reprogramming a la carte. Nat. Rev. Genet. 12, 231–242 (2011).
Aasen, T. et al. Efficient and rapid generation of induced pluripotent stem cells from human keratinocytes. Nat. Biotechnol. 26, 1276–1284 (2008).
Chin, M.H. et al. Induced pluripotent stem cells and embryonic stem cells are distinguished by gene expression signatures. Cell Stem Cell 5, 111–123 (2009).
Polo, J.M. et al. Cell type of origin influences the molecular and functional properties of mouse induced pluripotent stem cells. Nat. Biotechnol. 28, 848–855 (2010).
Ohi, Y. et al. Incomplete DNA methylation underlies a transcriptional memory of somatic cells in human iPS cells. Nat. Cell Biol. 13, 541–549 (2011).
Bar-Nur, O., Russ, H.A., Efrat, S. & Benvenisty, N. Epigenetic memory and preferential lineage-specific differentiation in induced pluripotent stem cells derived from human pancreatic islet beta cells. Cell Stem Cell 9, 17–23 (2011).
Kim, K. et al. Epigenetic memory in induced pluripotent stem cells. Nature 467, 285–290 (2010).
Stadtfeld, M. et al. Aberrant silencing of imprinted genes on chromosome 12qF1 in mouse induced pluripotent stem cells. Nature 465, 175–181 (2010).
Maherali, N. & Hochedlinger, K. Guidelines and techniques for the generation of induced pluripotent stem cells. Cell Stem Cell 3, 595–605 (2008).
Irion, S., Nostro, M.C., Kattman, S.J. & Keller, G.M. Directed differentiation of pluripotent stem cells: from developmental biology to therapeutic applications. Cold Spring Harb. Symp. Quant. Biol. 73, 101–110 (2008).
Murry, C.E. & Keller, G. Differentiation of embryonic stem cells to clinically relevant populations: lessons from embryonic development. Cell 132, 661–680 (2008).
Moretti, A. et al. Patient-specific induced pluripotent stem-cell models for long-QT syndrome. N. Engl. J. Med. 363, 1397–1409 (2010).
Yazawa, M. et al. Using induced pluripotent stem cells to investigate cardiac phenotypes in Timothy syndrome. Nature 471, 230–234 (2011).
Zhang, J. et al. A human iPSC model of Hutchinson Gilford Progeria reveals vascular smooth muscle and mesenchymal stem cell defects. Cell Stem Cell 8, 31–45 (2011).
Liu, G.H. et al. Targeted gene correction of laminopathy-associated LMNA mutations in patient-specific iPSCs. Cell Stem Cell 8, 688–694 (2011).
Saha, K. & Jaenisch, R. Technical challenges in using human induced pluripotent stem cells to model disease. Cell Stem Cell 5, 584–595 (2009).
Zhu, H., Lensch, M.W., Cahan, P. & Daley, G.Q. Investigating monogenic and complex diseases with pluripotent stem cells. Nat. Rev. Genet. 12, 266–275 (2011).
Nguyen, H.N. et al. LRRK2 mutant iPSC–derived DA neurons demonstrate increased susceptibility to oxidative stress. Cell Stem Cell 8, 267–280 (2011).
Agarwal, S. et al. Telomere elongation in induced pluripotent stem cells from dyskeratosis congenita patients. Nature 464, 292–296 (2010).
Acknowledgements
The authors thank R. Vassena and M. Barragan Monasterio for their critical reading of the manuscript. Work in the laboratory of J.C.I.B. was funded by Ramon y Cajal, The Helmsley Charitable Trust, Sanofi-Aventis, The G. Harold and Leila Y. Mathers Charitable Foundation and Fundacion Cellex.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Tiscornia, G., Vivas, E. & Belmonte, J. Diseases in a dish: modeling human genetic disorders using induced pluripotent cells. Nat Med 17, 1570–1576 (2011). https://doi.org/10.1038/nm.2504
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.2504
This article is cited by
-
Characterization of an iPSC-based barrier model for blood-brain barrier investigations using the SBAD0201 stem cell line
Fluids and Barriers of the CNS (2023)
-
Germline PTEN genotype-dependent phenotypic divergence during the early neural developmental process of forebrain organoids
Molecular Psychiatry (2023)
-
Thinking in 3 dimensions: philosophies of the microenvironment in organoids and organs-on-chip
History and Philosophy of the Life Sciences (2023)
-
Recapitulation of pro-inflammatory signature of monocytes with ACVR1A mutation using FOP patient-derived iPSCs
Orphanet Journal of Rare Diseases (2022)
-
Developmental modeling of hepatogenesis using obese iPSCs-hepatocyte differentiation uncovers pathological features
Cell Death & Disease (2022)