Abstract
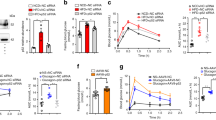
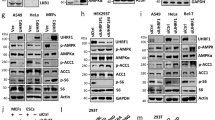
Here we show that p38 mitogen-activated protein kinase (p38 MAPK) phosphorylates the spliced form of X-box binding protein 1 (Xbp1s) on its Thr48 and Ser61 residues and greatly enhances its nuclear migration in mice, whereas mutation of either residue to alanine substantially reduces its nuclear translocation and activity. We also show that p38 MAPK activity is markedly reduced in the livers of obese mice compared with lean mice. Further, we show that activation of p38 MAPK by expression of constitutively active MAP kinase kinase 6 (MKK6Glu) greatly enhances nuclear translocation of Xbp1s, reduces endoplasmic reticulum stress and establishes euglycemia in severely obese and diabetic mice. Hence, our results define a crucial role for phosphorylation on Thr48 and Ser61 of Xbp1s in the maintenance of glucose homeostasis in obesity, and they suggest that p38 MAPK activation in the livers of obese mice could lead to a new therapeutic approach to the treatment of type 2 diabetes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Marciniak, S.J. & Ron, D. Endoplasmic reticulum stress signaling in disease. Physiol. Rev. 86, 1133–1149 (2006).
Schröder, M. & Kaufman, R.J. The mammalian unfolded protein response. Annu. Rev. Biochem. 74, 739–789 (2005).
Ron, D. & Walter, P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat. Rev. Mol. Cell Biol. 8, 519–529 (2007).
Bernales, S., Papa, F.R. & Walter, P. Intracellular signaling by the unfolded protein response. Annu. Rev. Cell Dev. Biol. 22, 487–508 (2006).
Cox, J.S., Shamu, C.E. & Walter, P. Transcriptional induction of genes encoding endoplasmic reticulum resident proteins requires a transmembrane protein kinase. Cell 73, 1197–1206 (1993).
Mori, K., Ma, W., Gething, M.J. & Sambrook, J. A transmembrane protein with a cdc2+/CDC28-related kinase activity is required for signaling from the ER to the nucleus. Cell 74, 743–756 (1993).
Yoshida, H., Matsui, T., Yamamoto, A., Okada, T. & Mori, K. XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell 107, 881–891 (2001).
Lee, K. et al. IRE1-mediated unconventional mRNA splicing and S2P-mediated ATF6 cleavage merge to regulate XBP1 in signaling the unfolded protein response. Genes Dev. 16, 452–466 (2002).
Calfon, M. et al. IRE1 couples endoplasmic reticulum load to secretory capacity by processing the XBP–1 mRNA. Nature 415, 92–96 (2002).
Clauss, I.M., Chu, M., Zhao, J.L. & Glimcher, L.H. The basic domain/leucine zipper protein hXBP–1 preferentially binds to and transactivates CRE-like sequences containing an ACGT core. Nucleic Acids Res. 24, 1855–1864 (1996).
Lee, A.H., Iwakoshi, N.N. & Glimcher, L.H. XBP–1 regulates a subset of endoplasmic reticulum resident chaperone genes in the unfolded protein response. Mol. Cell. Biol. 23, 7448–7459 (2003).
Sriburi, R., Jackowski, S., Mori, K. & Brewer, J.W. XBP1: a link between the unfolded protein response, lipid biosynthesis, and biogenesis of the endoplasmic reticulum. J. Cell Biol. 167, 35–41 (2004).
Sriburi, R. et al. Coordinate regulation of phospholipid biosynthesis and secretory pathway gene expression in XBP–1(S)-induced endoplasmic reticulum biogenesis. J. Biol. Chem. 282, 7024–7034 (2007).
Fagone, P. et al. Phospholipid biosynthesis program underlying membrane expansion during B-lymphocyte differentiation. J. Biol. Chem. 282, 7591–7605 (2007).
Ozcan, U. et al. Loss of the tuberous sclerosis complex tumor suppressors triggers the unfolded protein response to regulate insulin signaling and apoptosis. Mol. Cell 29, 541–551 (2008).
Ozcan, U. et al. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science 313, 1137–1140 (2006).
Ozcan, U. et al. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science 306, 457–461 (2004).
Ozcan, L. et al. Endoplasmic reticulum stress plays a central role in development of leptin resistance. Cell Metab. 9, 35–51 (2009).
Richardson, C.E., Kooistra, T. & Kim, D.H. An essential role for XBP–1 in host protection against immune activation in C. elegans. Nature 463, 1092–1095 (2010).
Martinon, F., Chen, X., Lee, A.H. & Glimcher, L.H. TLR activation of the transcription factor XBP1 regulates innate immune responses in macrophages. Nat. Immunol. 11, 411–418 (2010).
Sado, M. et al. Protective effect against Parkinson′s disease-related insults through the activation of XBP1. Brain Res. 1257, 16–24 (2009).
Kaser, A. et al. XBP1 links ER stress to intestinal inflammation and confers genetic risk for human inflammatory bowel disease. Cell 134, 743–756 (2008).
Koong, A.C., Chauhan, V. & Romero-Ramirez, L. Targeting XBP–1 as a novel anti-cancer strategy. Cancer Biol. Ther. 5, 756–759 (2006).
Nakatani, Y. et al. Involvement of endoplasmic reticulum stress in insulin resistance and diabetes. J. Biol. Chem. 280, 847–851 (2005).
Ozawa, K. et al. The endoplasmic reticulum chaperone improves insulin resistance in type 2 diabetes. Diabetes 54, 657–663 (2005).
Park, S.W. et al. The regulatory subunits of PI3K, p85alpha and p85beta, interact with XBP–1 and increase its nuclear translocation. Nat. Med. 16, 429–437 (2010).
Zhou, Y. et al. Regulation of glucose homeostasis through a XBP–1-FoxO1 interaction. Nat. Med. 17, 356–365 (2011).
Coulthard, L.R., White, D.E., Jones, D.L., McDermott, M.F. & Burchill, S.A. p38(MAPK): stress responses from molecular mechanisms to therapeutics. Trends Mol. Med. 15, 369–379 (2009).
Morrison, D.K. & Davis, R.J. Regulation of MAP kinase signaling modules by scaffold proteins in mammals. Annu. Rev. Cell Dev. Biol. 19, 91–118 (2003).
Chang, L. & Karin, M. Mammalian MAP kinase signalling cascades. Nature 410, 37–40 (2001).
Dong, C., Davis, R.J. & Flavell, R.A. MAP kinases in the immune response. Annu. Rev. Immunol. 20, 55–72 (2002).
Urano, F. et al. Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science 287, 664–666 (2000).
Matsuzawa, A., Nishitoh, H., Tobiume, K., Takeda, K. & Ichijo, H. Physiological roles of ASK1-mediated signal transduction in oxidative stress- and endoplasmic reticulum stress-induced apoptosis: advanced findings from ASK1 knockout mice. Antioxid. Redox Signal. 4, 415–425 (2002).
Park, S.K., Sanders, B.G. & Kline, K. Tocotrienols induce apoptosis in breast cancer cell lines via an endoplasmic reticulum stress-dependent increase in extrinsic death receptor signaling. Breast Cancer Res. Treat. 124, 361–375 (2010).
Lin, J.H. et al. IRE1 signaling affects cell fate during the unfolded protein response. Science 318, 944–949 (2007).
Tournier, C. et al. MKK7 is an essential component of the JNK signal transduction pathway activated by proinflammatory cytokines. Genes Dev. 15, 1419–1426 (2001).
Kyriakis, J.M. & Avruch, J. Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol. Rev. 81, 807–869 (2001).
Schaeffer, H.J. & Weber, M.J. Mitogen-activated protein kinases: specific messages from ubiquitous messengers. Mol. Cell. Biol. 19, 2435–2444 (1999).
Lei, K. et al. The Bax subfamily of Bcl2-related proteins is essential for apoptotic signal transduction by c-Jun NH(2)-terminal kinase. Mol. Cell. Biol. 22, 4929–4942 (2002).
Brancho, D. et al. Mechanism of p38 MAP kinase activation in vivo. Genes Dev. 17, 1969–1978 (2003).
Raingeaud, J., Whitmarsh, A.J., Barrett, T., Derijard, B. & Davis, R.J. MKK3- and MKK6-regulated gene expression is mediated by the p38 mitogen-activated protein kinase signal transduction pathway. Mol. Cell. Biol. 16, 1247–1255 (1996).
Clark, A., Dean, J., Tudor, C. & Saklatvala, J. Post-transcriptional gene regulation by MAP kinases via AU-rich elements. Front. Biosci. 14, 847–871 (2009).
Park, E.J. et al. Dietary and genetic obesity promote liver inflammation and tumorigenesis by enhancing IL-6 and TNF expression. Cell 140, 197–208 (2010).
Olefsky, J.M. & Glass, C.K. Macrophages, inflammation, and insulin resistance. Annu. Rev. Physiol. 72, 219–246 (2010).
Shoelson, S.E., Lee, J. & Goldfine, A.B. Inflammation and insulin resistance. J. Clin. Invest. 116, 1793–1801 (2006).
Solinas, G. & Karin, M. JNK1 and IKKbeta: molecular links between obesity and metabolic dysfunction. FASEB J. 24, 2596–2611 (2010).
Arkan, M.C. et al. IKK-beta links inflammation to obesity-induced insulin resistance. Nat. Med. 11, 191–198 (2005).
Solinas, G. et al. JNK1 in hematopoietically derived cells contributes to diet-induced inflammation and insulin resistance without affecting obesity. Cell Metab. 6, 386–397 (2007).
Hirosumi, J. et al. A central role for JNK in obesity and insulin resistance. Nature 420, 333–336 (2002).
Lee, A.H., Iwakoshi, N.N., Anderson, K.C. & Glimcher, L.H. Proteasome inhibitors disrupt the unfolded protein response in myeloma cells. Proc. Natl. Acad. Sci. USA 100, 9946–9951 (2003).
Acknowledgements
We thank members of the Ozcan laboratory for their help during the execution of the experiments. We thank L. Glimcher (Harvard School of Public Health) for providing us with the Xbp1flox/flox mouse strain. We thank P. Blackshear (National Institute of Environment Health Sciences) for kindly providing us with Zfp36−/− cells and M. Gaestel (Hannover Medical School, Germany) for generously providing Mapkapk2−/− cells. We thank R. Davis (University of Massachusetts Medical School) for providing Mapk8−/−; Mapk9−/−, Map2k3−/−; Map2k6−/−, Map2k4−/−; Map2k7−/− and Mapk14−/− MEFs as well as their wild-type control MEFs. We are grateful to H. Feldman (Harvard Medical School) for helping us with the statistical analysis. This study was supported by the junior faculty start-up funds provided to U.O. by Children's Hospital Boston, an RO1 grant (R01DK081009) and R56 grant (R56DK089111) provided to U.O. by the US National Institutes of Health and the Timothy Murphy funds provided to the Division of Endocrinology, Children's Hospital Boston.
Author information
Authors and Affiliations
Contributions
Jaemin Lee and C.S. designed and carried out the experiments, analyzed the data and wrote the manuscript. Y.Z., Justin Lee, D.G., H.H., S.W.P. did the experiments. R.J.D. provided reagents and advice through out the project. U.O. developed the hypothesis, designed experiments, analyzed the data and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–7 and Supplementary Methods (PDF 1648 kb)
Rights and permissions
About this article
Cite this article
Lee, J., Sun, C., Zhou, Y. et al. p38 MAPK–mediated regulation of Xbp1s is crucial for glucose homeostasis. Nat Med 17, 1251–1260 (2011). https://doi.org/10.1038/nm.2449
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.2449
This article is cited by
-
MKP1 promotes nonalcoholic steatohepatitis by suppressing AMPK activity through LKB1 nuclear retention
Nature Communications (2023)
-
Akaluc/AkaLumine bioluminescence system enables highly sensitive, non-invasive and temporal monitoring of gene expression in Drosophila
Communications Biology (2023)
-
The activation of spliced X-box binding protein 1 by isorhynchophylline therapy improves diabetic encephalopathy
Cell Biology and Toxicology (2023)
-
Utility of the burmese Python as a model for studying plasticity of extreme physiological systems
Journal of Muscle Research and Cell Motility (2023)
-
Food odor perception promotes systemic lipid utilization
Nature Metabolism (2022)