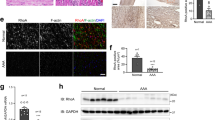
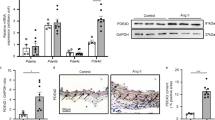
Abstract
Hypertension is one of the most frequent pathologies in the industrialized world. Although recognized to be dependent on a combination of genetic and environmental factors, its molecular basis remains elusive. Increased activity of the monomeric G protein RhoA in arteries is a common feature of hypertension. However, how RhoA is activated and whether it has a causative role in hypertension remains unclear. Here we provide evidence that Arhgef1 is the RhoA guanine exchange factor specifically responsible for angiotensin II–induced activation of RhoA signaling in arterial smooth muscle cells. We found that angiotensin II activates Arhgef1 through a previously undescribed mechanism in which Jak2 phosphorylates Tyr738 of Arhgef1. Arhgef1 inactivation in smooth muscle induced resistance to angiotensin II–dependent hypertension in mice, but did not affect normal blood pressure regulation. Our results show that control of RhoA signaling through Arhgef1 is central to the development of angiotensin II–dependent hypertension and identify Arhgef1 as a potential target for the treatment of hypertension.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Staessen, J.A., Wang, J., Bianchi, G. & Birkenhager, W.H. Essential hypertension. Lancet 361, 1629–1641 (2003).
de Gasparo, M., Catt, K.J., Inagami, T., Wright, J.W. & Unger, T. International union of pharmacology. XXIII. The angiotensin II receptors. Pharmacol. Rev. 52, 415–472 (2000).
Touyz, R.M. & Berry, C. Recent advances in angiotensin II signaling. Braz. J. Med. Biol. Res. 35, 1001–1015 (2002).
Chrissobolis, S. & Sobey, C.G. Evidence that Rho-kinase activity contributes to cerebral vascular tone in vivo and is enhanced during chronic hypertension: comparison with protein kinase C. Circ. Res. 88, 774–779 (2001).
Mukai, Y. et al. Involvement of Rho-kinase in hypertensive vascular disease: a novel therapeutic target in hypertension. FASEB J. 15, 1062–1064 (2001).
Seko, T. et al. Activation of RhoA and inhibition of myosin phosphatase as important components in hypertension in vascular smooth muscle. Circ. Res. 92, 411–418 (2003).
Uehata, M. et al. Calcium sensitization of smooth muscle mediated by a Rho-associated protein kinase in hypertension. Nature 389, 990–994 (1997).
Masumoto, A. et al. Possible involvement of Rho-kinase in the pathogenesis of hypertension in humans. Hypertension 38, 1307–1310 (2001).
Jaffe, A.B. & Hall, A. Rho GTPases: biochemistry and biology. Annu. Rev. Cell Dev. Biol. 21, 247–269 (2005).
Somlyo, A.P. & Somlyo, A.V. Ca2+ sensitivity of smooth muscle and nonmuscle myosin II: modulated by G proteins, kinases, and myosin phosphatase. Physiol. Rev. 83, 1325–1358 (2003).
Loirand, G., Guerin, P. & Pacaud, P. Rho kinases in cardiovascular physiology and pathophysiology. Circ. Res. 98, 322–334 (2006).
Kataoka, C. et al. Important role of Rho-kinase in the pathogenesis of cardiovascular inflammation and remodeling induced by long-term blockade of nitric oxide synthesis in rats. Hypertension 39, 245–250 (2002).
Moriki, N. et al. RhoA activation in vascular smooth muscle cells from stroke-prone spontaneously hypertensive rats. Hypertens. Res. 27, 263–270 (2004).
Higashi, M. et al. Long-term inhibition of Rho-kinase suppresses angiotensin II-induced cardiovascular hypertrophy in rats in vivo: effect on endothelial NAD(P)H oxidase system. Circ. Res. 93, 767–775 (2003).
Rossman, K.L., Der, C.J. & Sondek, J. GEF means go: turning on RHO GTPases with guanine nucleotide-exchange factors. Nat. Rev. Mol. Cell Biol. 6, 167–180 (2005).
Bos, J.L., Rehmann, H. & Wittinghofer, A. GEFs and GAPs: critical elements in the control of small G proteins. Cell 129, 865–877 (2007).
Garcia-Mata, R. et al. Analysis of activated GAPs and GEFs in cell lysates. Methods Enzymol. 406, 425–437 (2006).
Mehta, P.K. & Griendling, K.K. Angiotensin II cell signaling: physiological and pathological effects in the cardiovascular system. Am. J. Physiol. Cell Physiol. 292, C82–C97 (2007).
Rubtsov, A. et al. Lsc regulates marginal-zone B cell migration and adhesion and is required for the IgM T-dependent antibody response. Immunity 23, 527–538 (2005).
Wirth, A. et al. G12–G13-LARG-mediated signaling in vascular smooth muscle is required for salt-induced hypertension. Nat. Med. 14, 64–68 (2008).
Fukuhara, S., Murga, C., Zohar, M., Igishi, T. & Gutkind, J.S. A novel PDZ domain containing guanine nucleotide exchange factor links heterotrimeric G proteins to Rho. J. Biol. Chem. 274, 5868–5879 (1999).
Kozasa, T. et al. p115 RhoGEF, a GTPase activating protein for Galpha12 and Galpha13. Science 280, 2109–2111 (1998).
Suzuki, N., Nakamura, S., Mano, H. & Kozasa, T. Galpha 12 activates Rho GTPase through tyrosine-phosphorylated leukemia-associated RhoGEF. Proc. Natl. Acad. Sci. USA 100, 733–738 (2003).
Fukuhara, S., Chikumi, H. & Gutkind, J.S. Leukemia-associated Rho guanine nucleotide exchange factor (LARG) links heterotrimeric G proteins of the G(12) family to Rho. FEBS Lett. 485, 183–188 (2000).
Wells, C.D. et al. Mechanisms for reversible regulation between G13 and Rho exchange factors. J. Biol. Chem. 277, 1174–1181 (2002).
Frank, G.D. et al. Requirement of Ca(2+) and PKCdelta for Janus kinase 2 activation by angiotensin II: involvement of PYK2. Mol. Endocrinol. 16, 367–377 (2002).
Marrero, M.B. et al. Direct stimulation of Jak/STAT pathway by the angiotensin II AT1 receptor. Nature 375, 247–250 (1995).
Ohtsu, H. et al. Central role of Gq in the hypertrophic signal transduction of angiotensin II in vascular smooth muscle cells. Endocrinology 149, 3569–3575 (2008).
Keys, J.R., Greene, E.A., Koch, W.J. & Eckhart, A.D. Gq-coupled receptor agonists mediate cardiac hypertrophy via the vasculature. Hypertension 40, 660–666 (2002).
Marrero, M.B. et al. Role of Janus kinase/signal transducer and activator of transcription and mitogen-activated protein kinase cascades in angiotensin II- and platelet-derived growth factor-induced vascular smooth muscle cell proliferation. J. Biol. Chem. 272, 24684–24690 (1997).
Shaw, S. et al. High glucose augments the angiotensin II-induced activation of JAK2 in vascular smooth muscle cells via the polyol pathway. J. Biol. Chem. 278, 30634–30641 (2003).
Banes-Berceli, A.K. et al. Angiotensin II and endothelin-1 augment the vascular complications of diabetes via JAK2 activation. Am. J. Physiol. Heart Circ. Physiol. 293, H1291–H1299 (2007).
Garcia-Mata, R. & Burridge, K. Catching a GEF by its tail. Trends Cell Biol. 17, 36–43 (2007).
Fujii, A.M. & Vatner, S.F. Direct versus indirect pressor and vasoconstrictor actions of angiotensin in conscious dogs. Hypertension 7, 253–261 (1985).
Rowe, B.P., Noble, A.R. & Munday, K.A. Blockade of pressor responses to angiotensins I and II and noradrenaline using phentolamine, propranolol and hexamethonium in conscious rabbits. Pflugers Arch. 382, 269–274 (1979).
Cline, W.H. Jr. Role of released catecholamines in the vascular response to injected angiotensin II in the dog. J. Pharmacol. Exp. Ther. 216, 104–110 (1981).
Ferrario, C.M., Barnes, K.L., Szilagyi, J.E. & Brosnihan, K.B. Physiological and pharmacological characterization of the area postrema pressor pathways in the normal dog. Hypertension 1, 235–245 (1979).
Falcon, J.C. II, Phillips, M.I., Hoffman, W.E. & Brody, M.J. Effects of intraventricular angiotensin II mediated by the sympathetic nervous system. Am. J. Physiol. 235, H392–H399 (1978).
Crowley, S.D. et al. Angiotensin II causes hypertension and cardiac hypertrophy through its receptors in the kidney. Proc. Natl. Acad. Sci. USA 103, 17985–17990 (2006).
Ruilope, L.M., Lahera, V., Rodicio, J.L. & Carlos Romero, J. Are renal hemodynamics a key factor in the development and maintenance of arterial hypertension in humans? Hypertension 23, 3–9 (1994).
Schiffrin, E.L. Effects of aldosterone on the vasculature. Hypertension 47, 312–318 (2006).
Lemarie, C.A., Paradis, P. & Schiffrin, E.L. New insights on signaling cascades induced by cross-talk between angiotensin II and aldosterone. J. Mol. Med. 86, 673–678 (2008).
Montezano, A.C. et al. Aldosterone and angiotensin II synergistically stimulate migration in vascular smooth muscle cells through c-Src-regulated redox-sensitive RhoA pathways. Arterioscler. Thromb. Vasc. Biol. 28, 1511–1518 (2008).
Hein, L. et al. Overexpression of angiotensin AT1 receptor transgene in the mouse myocardium produces a lethal phenotype associated with myocyte hyperplasia and heart block. Proc. Natl. Acad. Sci. USA 94, 6391–6396 (1997).
Paradis, P., Dali-Youcef, N., Paradis, F.W., Thibault, G. & Nemer, M. Overexpression of angiotensin II type I receptor in cardiomyocytes induces cardiac hypertrophy and remodeling. Proc. Natl. Acad. Sci. USA 97, 931–936 (2000).
Struthers, A.D. & MacDonald, T.M. Review of aldosterone- and angiotensin II-induced target organ damage and prevention. Cardiovasc. Res. 61, 663–670 (2004).
Feng, J. et al. Inhibitory phosphorylation site for Rho-associated kinase on smooth muscle myosin phosphatase. J. Biol. Chem. 274, 37385–37390 (1999).
Ren, X.D., Kiosses, W.B. & Schwartz, M.A. Regulation of the small GTP-binding protein Rho by cell adhesion and the cytoskeleton. EMBO J. 18, 578–585 (1999).
Mills, P.A. et al. A new method for measurement of blood pressure, heart rate, and activity in the mouse by radiotelemetry. J. Appl. Physiol. 88, 1537–1544 (2000).
Krege, J.H., Hodgin, J.B., Hagaman, J.R. & Smithies, O. A noninvasive computerized tail-cuff system for measuring blood pressure in mice. Hypertension 25, 1111–1115 (1995).
Acknowledgements
The authors are grateful to K. Burridge (University of North Carolina, Chapel Hill), V. Perrot (University of Rouen) and J. Ihle (St. Jude Children's Research Hospital, Memphis) for providing us with GST-RhoA constructs, Arhgef1 and Jak2 plasmids, respectively. We thank C. Schleder, N. Vaillant and M. Rio for excellent technical assistance and the IBISA platform Cardiex for functional explorations. This work was supported by the Institut National de la Santé et de la Recherche Médicale, the Agence Nationale de la Recherche (ANR-05-PCOD-015-01 and ANR-08-GENO-040-01), the Fondation pour la Recherche Médicale (Dequation (20051205767)), the Programme National de Recherche Maladies Cardiovasculaires and the Société Française d'hypertension artérielle (PNRC 2007), the Centre National de la Recherche Scientifique (Groupement de Recherche 2823) and the Institut de Recherches Servier.
Author information
Authors and Affiliations
Contributions
C.G. contributed to study design and performed all experiments with J.B. G.T. and M.R.-D. collaborated on in vitro experiments, proteomics and pulldown analyses. K.R., L.L. and D.H. collaborated on ex vivo contraction measurements. E.S. and A.B. helped with the organization of the study. R.M.T. and S.O. generated Arhgef1lox/lox mice and SMMHC-CreERT2 mice, respectively. P.P. and G.L. planned and directed the study and wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–7 and Supplementary Tables 1–2 (PDF 868 kb)
Rights and permissions
About this article
Cite this article
Guilluy, C., Brégeon, J., Toumaniantz, G. et al. The Rho exchange factor Arhgef1 mediates the effects of angiotensin II on vascular tone and blood pressure. Nat Med 16, 183–190 (2010). https://doi.org/10.1038/nm.2079
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.2079
This article is cited by
-
Enhanced BCAT1 activity and BCAA metabolism promotes RhoC activity in cancer progression
Nature Metabolism (2023)
-
Regulation of myosin light-chain phosphorylation and its roles in cardiovascular physiology and pathophysiology
Hypertension Research (2022)
-
Kidney and epigenetic mechanisms of salt-sensitive hypertension
Nature Reviews Nephrology (2021)
-
Tyrosine phosphatase Shp2 regulates p115RhoGEF/Rho-dependent dendritic cell migration
Cellular & Molecular Immunology (2021)
-
Regulation of heterogeneous cancer-associated fibroblasts: the molecular pathology of activated signaling pathways
Journal of Experimental & Clinical Cancer Research (2020)