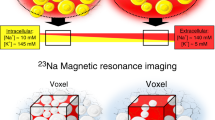
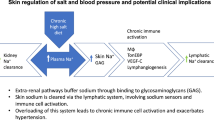
Abstract
In salt-sensitive hypertension, the accumulation of Na+ in tissue has been presumed to be accompanied by a commensurate retention of water to maintain the isotonicity of body fluids. We show here that a high-salt diet (HSD) in rats leads to interstitial hypertonic Na+ accumulation in skin, resulting in increased density and hyperplasia of the lymphcapillary network. The mechanisms underlying these effects on lymphatics involve activation of tonicity-responsive enhancer binding protein (TonEBP) in mononuclear phagocyte system (MPS) cells infiltrating the interstitium of the skin. TonEBP binds the promoter of the gene encoding vascular endothelial growth factor-C (VEGF-C, encoded by Vegfc) and causes VEGF-C secretion by macrophages. MPS cell depletion or VEGF-C trapping by soluble VEGF receptor-3 blocks VEGF-C signaling, augments interstitial hypertonic volume retention, decreases endothelial nitric oxide synthase expression and elevates blood pressure in response to HSD. Our data show that TonEBP–VEGF-C signaling in MPS cells is a major determinant of extracellular volume and blood pressure homeostasis and identify VEGFC as an osmosensitive, hypertonicity-driven gene intimately involved in salt-induced hypertension.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Adrogué, H.J. & Madias, N.E. Sodium and potassium in the pathogenesis of hypertension. N. Engl. J. Med. 356, 1966–1978 (2007).
Guyton, A.C. et al. Systems analysis of arterial pressure regulation and hypertension. Ann. Biomed. Eng. 1, 254–281 (1972).
Lifton, R.P., Gharavi, A.G. & Geller, D.S. Molecular mechanisms of human hypertension. Cell 104, 545–556 (2001).
Pitts, R.F. in Physiology of the kidney and body fluids (ed. Pitts, R. F.) 11–34 (Year Book Medical Publishers, Chicago, 1974).
Heer, M., Baisch, F., Kropp, J., Gerzer, R. & Drummer, C. High dietary sodium chloride consumption may not induce body fluid retention in humans. Am. J. Physiol. Renal Physiol. 278, F585–F595 (2000).
Titze, J. et al. Long-term sodium balance in humans in a terrestrial space station simulation study. Am. J. Kidney Dis. 40, 508–516 (2002).
Titze, J. et al. Internal sodium balance in DOCA-salt rats: a body composition study. Am. J. Physiol. Renal Physiol. 289, F793–F802 (2005).
Titze, J. et al. Osmotically inactive skin Na+ storage in rats. Am. J. Physiol. Renal Physiol. 285, F1108–F1117 (2003).
Ziomber, A. et al. Sodium-, potassium-, chloride- and bicarbonate-related effects on blood pressure and electrolyte homeostasis in deoxycorticosterone acetate-treated rats. Am. J. Physiol. Renal Physiol. 295, F1752–F1763 (2008).
Go, W.Y., Liu, X., Roti, M.A., Liu, F. & Ho, S.N. NFAT5/TonEBP mutant mice define osmotic stress as a critical feature of the lymphoid microenvironment. Proc. Natl. Acad. Sci. USA 101, 10673–10678 (2004).
Schafflhuber, M. et al. Mobilization of osmotically inactive Na+ by growth and by dietary salt restriction in rats. Am. J. Physiol. Renal Physiol. 292, F1490–F1500 (2007).
Titze, J. et al. Glycosaminoglycan polymerization may enable osmotically inactive Na+ storage in the skin. Am. J. Physiol. Heart Circ. Physiol. 287, H203–H208 (2004).
Fukuda, S., Yasu, T., Kobayashi, N., Ikeda, N. & Schmid-Schonbein, G.W. Contribution of fluid shear response in leukocytes to hemodynamic resistance in the spontaneously hypertensive rat. Circ. Res. 95, 100–108 (2004).
Guzik, T.J. et al. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J. Exp. Med. 204, 2449–2460 (2007).
Jeltsch, M. et al. Hyperplasia of lymphatic vessels in VEGF-C transgenic mice. Science 276, 1423–1425 (1997).
Kerjaschki, D. The crucial role of macrophages in lymphangiogenesis. J. Clin. Invest. 115, 2316–2319 (2005).
Schoppmann, S.F. et al. Tumor-associated macrophages express lymphatic endothelial growth factors and are related to peritumoral lymphangiogenesis. Am. J. Pathol. 161, 947–956 (2002).
Lohela, M., Helotera, H., Haiko, P., Dumont, D.J. & Alitalo, K. Transgenic induction of vascular endothelial growth factor-C is strongly angiogenic in mouse embryos but leads to persistent lymphatic hyperplasia in adult tissues. Am. J. Pathol. 173, 1891–1901 (2008).
Miyakawa, H., Woo, S.K., Dahl, S.C., Handler, J.S. & Kwon, H.M. Tonicity-responsive enhancer binding protein, a rel-like protein that stimulates transcription in response to hypertonicity. Proc. Natl. Acad. Sci. USA 96, 2538–2542 (1999).
Neuhofer, W. & Beck, F.X. Cell survial in the hostile environment of the renal medulla. Annu. Rev. Physiol. 67, 531–555 (2005).
Kang, K.T., Sullivan, J.C., Sasser, J.M., Imig, J.D. & Pollock, J.S. Novel nitric oxide synthase–dependent mechanism of vasorelaxation in small arteries from hypertensive rats. Hypertension 49, 893–901 (2007).
Leonard, A.M., Chafe, L.L., Montani, J.P. & Van Vliet, B.N. Increased salt-sensitivity in endothelial nitric oxide synthase–knockout mice. Am. J. Hypertens. 19, 1264–1269 (2006).
Tolins, J.P. & Shultz, P.J. Endogenous nitric oxide synthesis determines sensitivity to the pressor effect of salt. Kidney Int. 46, 230–236 (1994).
Joukov, V. et al. Proteolytic processing regulates receptor specificity and activity of VEGF-C. EMBO J. 16, 3898–3911 (1997).
Feng, Y., Venema, V.J., Venema, R.C., Tsai, N. & Caldwell, R.B. VEGF induces nuclear translocation of Flk-1/KDR, endothelial nitric oxide synthase, and caveolin-1 in vascular endothelial cells. Biochem. Biophys. Res. Commun. 256, 192–197 (1999).
He, H. et al. Vascular endothelial growth factor signals endothelial cell production of nitric oxide and prostacyclin through flk-1/KDR activation of c-Src. J. Biol. Chem. 274, 25130–25135 (1999).
Ahmad, S. et al. Direct evidence for endothelial vascular endothelial growth factor receptor-1 function in nitric oxide-mediated angiogenesis. Circ. Res. 99, 715–722 (2006).
Trzewik, J., Mallipattu, S.K., Artmann, G.M., Delano, F.A. & Schmid-Schonbein, G.W. Evidence for a second valve system in lymphatics: endothelial microvalves. FASEB J. 15, 1711–1717 (2001).
Boardman, K.C. & Swartz, M.A. Interstitial flow as a guide for lymphangiogenesis. Circ. Res. 92, 801–808 (2003).
Goldman, J. et al. Regulation of lymphatic capillary regeneration by interstitial flow in skin. Am. J. Physiol. Heart Circ. Physiol. 292, H2176–H2183 (2007).
Rutkowski, J.M., Boardman, K.C. & Swartz, M.A. Characterization of lymphangiogenesis in a model of adult skin regeneration. Am. J. Physiol. Heart Circ. Physiol. 291, H1402–H1410 (2006).
Konovchuk, V.N. Fiziol. Zh. SSSR Im. I M Sechenova [The participation of the central lymph in regulating water-salt metabolism and kidney function] 78, 42–47 (1992).
Oberleithner, H. et al. Plasma sodium stiffens vascular endothelium and reduces nitric oxide release. Proc. Natl. Acad. Sci. USA 104, 16281–16286 (2007).
Van Rooijen, N. & Sanders, A. Liposome mediated depletion of macrophages: mechanism of action, preparation of liposomes and applications. J. Immunol. Methods 174, 83–93 (1994).
He, Y. et al. Suppression of tumor lymphangiogenesis and lymph node metastasis by blocking vascular endothelial growth factor receptor 3 signaling. J. Natl. Cancer Inst. 94, 819–825 (2002).
Karpanen, T. et al. Vascular endothelial growth factor C promotes tumor lymphangiogenesis and intralymphatic tumor growth. Cancer Res. 61, 1786–1790 (2001).
Laitinen, M. et al. Adenovirus-mediated gene transfer to lower limb artery of patients with chronic critical leg ischemia. Hum. Gene Ther. 9, 1481–1486 (1998).
Jantsch, J. et al. Small interfering RNA (siRNA) delivery into murine bone marrow-derived dendritic cells by electroporation. J. Immunol. Methods 337, 71–77 (2008).
Miyakawa, H. et al. Cis- and trans-acting factors regulating transcription of the BGT1 gene in response to hypertonicity. Am. J. Physiol. 274, F753–F761 (1998).
Obst, M. et al. Nitric oxide synthase expression in AT2 receptor–deficient mice after DOCA-salt. Kidney Int. 65, 2268–2278 (2004).
Acknowledgements
This work was supported in part by grants from the Interdisziplinäres Zentrum für klinische Forschung Erlangen (TP B13), from the Bundesministerium für Bildung und Forschung - Forschung unter Weltraumbedingungen (50WB0620), and from the Deutsche Forschungsgemeinschaft (Ti345/2) to J.T., from the sixth Framework Integrated Project Lymphangiogenomics (LSGH-2004-503573) to D.K., and from a Fresenius Nephro-Core Stipend to A.Z. F.C.L. and D.N.M. were supported by EuReGene; D.N.M. is a Helmholtz fellow. We thank N. Rakova for translating Russian articles and E. Prell, M. Klewer and B. Hausknecht for their technical assistance. We thank P. Uhrin (Department of Vascular Biology and Thrombosis Research, Medical University of Vienna) for the Swiss-129Sv mice and H.M. Kwon (Department of Medicine, University of Maryland) for pCMV-Tag2-TonEBP.
Author information
Authors and Affiliations
Contributions
A.M. conducted the experiments and generated all experimental data, W.N. and F.-X.B. generated cells with stable TonEBP overexpression and contributed to the in vitro studies and writing of the manuscript, J.J. contributed to the in vitro studies, A.D., J.G. and A.Z. contributed to the animal studies, T.T. and K.A. provided adenoviruses and contributed to the adenoviral animal experiments, K.M. and A.K. did the three-dimensional resolution of the lymph capillary network, J.-K.P. analyzed and quantified eNOS expression, D.N.M. contributed to animal experiments and writing of the manuscript, W.D. provided human serum from subjects with refractory hypertension, P.D. and H.W. contributed to analysis of internal electrolyte redistribution in animal experiments by chemical analysis, N.v.R. generated clodronate liposomes for MPS depletion experiments, K.F.H. and K.-U.E. contributed to the conception of experimental design and the writing of the manuscript, F.C.L. provided serum samples from subjects and wrote the manuscript, D.K. contributed the methods and experimental design for quantification of lymph capillary network changes and the conception of macrophage–VEGF-C–lymph capillary interaction, J.T. planned and organized the experimental approach, supervised the project, analyzed the data statistically and wrote the manuscript.
Corresponding author
Supplementary information
Supplementary Text and Figures
Supplementary Figs. 1–4, Supplementary Table 1 and Supplementary Methods (PDF 775 kb)
Supplementary Video 1
Lymphcapillaries in rat ear, low-salt salt diet (MOV 4860 kb)
Supplementary Video 2
Lymphcapillaries in rat ear, high-salt diet (MOV 4086 kb)
Rights and permissions
About this article
Cite this article
Machnik, A., Neuhofer, W., Jantsch, J. et al. Macrophages regulate salt-dependent volume and blood pressure by a vascular endothelial growth factor-C–dependent buffering mechanism. Nat Med 15, 545–552 (2009). https://doi.org/10.1038/nm.1960
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.1960
This article is cited by
-
Immune and inflammatory mechanisms in hypertension
Nature Reviews Cardiology (2024)
-
A new approach to characterize cardiac sodium storage by combining fluorescence photometry and magnetic resonance imaging in small animal research
Scientific Reports (2024)
-
Differential impact of high-salt levels in vitro and in vivo on macrophage core functions
Molecular Biology Reports (2024)
-
Skin regulation of salt and blood pressure and potential clinical implications
Hypertension Research (2023)
-
Cancer- and infection-induced T cell exhaustion are distinct
Nature Immunology (2023)