Abstract
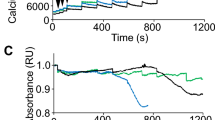
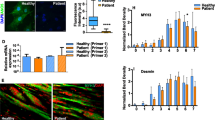
Duchenne muscular dystrophy is characterized by progressive muscle weakness and early death resulting from dystrophin deficiency. Loss of dystrophin results in disruption of a large dystrophin glycoprotein complex, leading to pathological calcium (Ca2+)-dependent signals that damage muscle cells1,2,3,4,5. We have identified a structural and functional defect in the ryanodine receptor (RyR1), a sarcoplasmic reticulum Ca2+ release channel, in the mdx mouse model of muscular dystrophy that contributes to altered Ca2+ homeostasis in dystrophic muscles. RyR1 isolated from mdx skeletal muscle showed an age-dependent increase in S-nitrosylation coincident with dystrophic changes in the muscle. RyR1 S-nitrosylation depleted the channel complex of FKBP12 (also known as calstabin-1, for calcium channel stabilizing binding protein), resulting in 'leaky' channels. Preventing calstabin-1 depletion from RyR1 with S107, a compound that binds the RyR1 channel and enhances the binding affinity of calstabin-1 to the nitrosylated channel, inhibited sarcoplasmic reticulum Ca2+ leak, reduced biochemical and histological evidence of muscle damage, improved muscle function and increased exercise performance in mdx mice. On the basis of these findings, we propose that sarcoplasmic reticulum Ca2+ leak via RyR1 due to S-nitrosylation of the channel and calstabin-1 depletion contributes to muscle weakness in muscular dystrophy, and that preventing the RyR1-mediated sarcoplasmic reticulum Ca2+ leak may provide a new therapeutic approach.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Bodensteiner, J.B. & Engel, A.G. Intracellular calcium accumulation in Duchenne dystrophy and other myopathies: a study of 567,000 muscle fibers in 114 biopsies. Neurology 28, 439–446 (1978).
Glesby, M.J., Rosenmann, E., Nylen, E.G. & Wrogemann, K. Serum CK, calcium, magnesium, and oxidative phosphorylation in mdx mouse muscular dystrophy. Muscle Nerve 11, 852–856 (1988).
Blake, D.J., Weir, A., Newey, S.E. & Davies, K.E. Function and genetics of dystrophin and dystrophin-related proteins in muscle. Physiol. Rev. 82, 291–329 (2002).
Turner, P.R., Westwood, T., Regen, C.M. & Steinhardt, R.A. Increased protein degradation results from elevated free calcium levels found in muscle from mdx mice. Nature 335, 735–738 (1988).
Fong, P.Y., Turner, P.R., Denetclaw, W.F. & Steinhardt, R.A. Increased activity of calcium leak channels in myotubes of Duchenne human and mdx mouse origin. Science 250, 673–676 (1990).
Hoffman, E.P., Brown, R.H. & Kunkel, L.M. Dystrophin: the protein product of the duchenne muscular dystrophy locus. Cell 51, 919–928 (1987).
Bonilla, E. et al. Duchenne muscular dystrophy: Deficiency of dystrophin at the muscle cell surface. Cell 54, 447–452 (1988).
Matsumura, K., Ervasti, J.M., Ohlendieck, K., Kahl, S.D. & Campbell, K.P. Association of dystrophin-related protein with dystrophin-associated proteins in mdx mouse muscle. Nature 360, 588–591 (1992).
Yeung, E.W. et al. Effects of stretch-activated channel blockers on [Ca2+]i and muscle damage in the mdx mouse. J. Physiol. (Lond.) 562, 367–380 (2005).
Bradley, W.G. & Fulthorpe, J.J. Studies of sarcolemmal integrity in myopathic muscle. Neurology 28, 670–677 (1978).
Franco, A., Jr. & Lansman, J.B. Calcium entry through stretch-inactivated ion channels in mdx myotubes. Nature 344, 670–673 (1990).
Vandebrouck, C., Martin, D., Colson-Van Schoor, M., Debaix, H. & Gailly, P. Involvement of TRPC in the abnormal calcium influx observed in dystrophic (mdx) mouse skeletal muscle fibers. J. Cell Biol. 158, 1089–1096 (2002).
Boittin, F.-X. et al. Ca2+-independent phospholipase A2 enhances store-operated Ca2+ entry in dystrophic skeletal muscle fibers. J. Cell Sci. 119, 3733–3742 (2006).
Robert, V. et al. Alteration in calcium handling at the subcellular level in mdx myotubes. J. Biol. Chem. 276, 4647–4651 (2001).
Spencer, M.J., Croall, D.E. & Tidball, J.G. Calpains are activated in necrotic fibers from mdx dystrophic mice. J. Biol. Chem. 270, 10909–10914 (1995).
Spencer, M.J. & Mellgren, R.L. Overexpression of a calpastatin transgene in mdx muscle reduces dystrophic pathology. Hum. Mol. Genet. 11, 2645–2655 (2002).
Turner, P.R., Fong, P.Y., Denetclaw, W.F. & Steinhardt, R.A. Increased calcium influx in dystrophic muscle. J. Cell Biol. 115, 1701–1712 (1991).
Wang, X. et al. Uncontrolled calcium sparks act as a dystrophic signal for mammalian skeletal muscle. Nat. Cell Biol. 7, 525–530 (2005).
Brenman, J.E., Chao, D.S., Xia, H., Aldape, K. & Bredt, D.S. Nitric oxide synthase complexed with dystrophin and absent from skeletal muscle sarcolemma in Duchenne muscular dystrophy. Cell 82, 743–752 (1995).
Chang, W.J. et al. Neuronal nitric oxide synthase and dystrophin-deficient muscular dystrophy. Proc. Natl. Acad. Sci. USA 93, 9142–9147 (1996).
Wehling, M., Spencer, M.J. & Tidball, J.G. A nitric oxide synthase transgene ameliorates muscular dystrophy in mdx mice. J. Cell Biol. 155, 123–131 (2001).
Xu, L., Eu, J.P., Meissner, G. & Stamler, J.S. Activation of the cardiac calcium release channel (ryanodine receptor) by poly-S-nitrosylation. Science 279, 234–237 (1998).
Sun, J., Xin, C., Eu, J.P., Stamler, J.S. & Meissner, G. Cysteine-3635 is responsible for skeletal muscle ryanodine receptor modulation by NO. Proc. Natl. Acad. Sci. USA 98, 11158–11162 (2001).
Aracena, P., Sanchez, G., Donoso, P., Hamilton, S.L. & Hidalgo, C. S-glutathionylation decreases Mg2+ inhibition and S-nitrosylation enhances Ca2+ activation of RyR1 channels. J. Biol. Chem. 278, 42927–42935 (2003).
Sun, J., Xu, L., Eu, J.P., Stamler, J.S. & Meissner, G. Nitric oxide, NOC-12, and S-nitrosoglutathione modulate the skeletal muscle calcium release channel/ryanodine Receptor by different mechanisms. An allosteric function for O2 in S-nitrosylation of the channel. J. Biol. Chem. 278, 8184–8189 (2003).
Aracena, P., Tang, W., Hamilton, S. & Hidalgo, C. Effects of S-glutathionylation and S-nitrosylation on calmodulin binding to triads and FKBP12 binding to type 1 calcium release channels. Antioxid. Redox Signal. 7, 870–881 (2005).
Marx, S.O. et al. Phosphorylation-dependent regulation of ryanodine receptors: a novel role for leucine/isoleucine zippers. J. Cell Biol. 153, 699–708 (2001).
Bellinger, A. et al. Remodeling of ryanodine receptor complex causes “leaky” channels: a molecular mechanism for decreased exercise capacity. Proc. Natl. Acad. Sci. USA 105, 2198–2202 (2008).
Wehrens, X.H. et al. Enhancing calstabin binding to ryanodine receptors improves cardiac and skeletal muscle function in heart failure. Proc. Natl. Acad. Sci. USA 102, 9607–9612 (2005).
Vilquin, J.T. et al. Evidence of mdx mouse skeletal muscle fragility in vivo by eccentric running exercise. Muscle Nerve 21, 567–576 (1998).
Brillantes, A.B. et al. Stabilization of calcium release channel (ryanodine receptor) function by FK506-binding protein. Cell 77, 513–523 (1994).
Marx, S.O., Ondrias, K. & Marks, A.R. Coupled gating between individual skeletal muscle Ca2+ release channels (ryanodine receptors). Science 281, 818–821 (1998).
Kameya, S. α1-syntrophin gene disruption results in the absence of neuronal-type nitric-oxide synthase at the sarcolemma but does not induce muscle degeneration. J. Biol. Chem. 274, 2193–2200 (1999).
Kobayashi, Y.M. et al. Sarcolemma-localized nNOS is required to maintain activity after mild exercise. Nature 456, 511–515 (2008).
Louboutin, J.P., Rouger, K., Tinsley, J.M., Halldorson, J. & Wilson, J.M. iNOS expression in dystrophinopathies can be reduced by somatic gene transfer of dystrophin or utrophin. Mol. Med. 7, 355–364 (2001).
Gregorevic, P. et al. rAAV6-microdystrophin preserves muscle function and extends lifespan in severely dystrophic mice. Nat. Med. 12, 787–789 (2006).
Krag, T.O.B. et al. Heregulin ameliorates the dystrophic phenotype in mdx mice. Proc. Natl. Acad. Sci. USA 101, 13856–13860 (2004).
Zaccagnini, G. et al. p66ShcA and oxidative stress modulate myogenic differentiation and skeletal muscle regeneration after hindlimb ischemia. J. Biol. Chem. 282, 31453–31459 (2007).
Khurana, T.S. & Davies, K.E. Pharmacological strategies for muscular dystrophy. Nat. Rev. Drug Discov. 2, 379–390 (2003).
Goyenvalle, A. et al. Rescue of dystrophic muscle through U7 snRNA-mediated exon skipping. Science 306, 1796–1799 (2004).
Marx, S.O. et al. PKA phosphorylation dissociates FKBP12.6 from the calcium release channel (ryanodine receptor): defective regulation in failing hearts. Cell 101, 365–376 (2000).
Reiken, S. et al. PKA phosphorylation activates the calcium release channel (ryanodine receptor) in skeletal muscle: defective regulation in heart failure. J. Cell. Biol. 160, 919–928 (2003).
Acknowledgements
This work was supported in part by a grant from the Leducq Foundation. We thank J. Shan for assistance with analyses of histologic sections and J. Fauconnier for help with voluntary exercise measurements in mice.
Author information
Authors and Affiliations
Contributions
A.M.B. conducted experiments and wrote the manuscript, S.R. performed biochemistry experiments, C.C. assisted with mouse experiments, M.M. performed immunohistochemistry, X.L. and L.R. assisted with histology, S.M. and A.L. performed muscle and calcium experiments, and A.R.M. conceived, designed and directed the project, analyzed data and wrote the final version of the manuscript.
Corresponding author
Supplementary information
Supplementary Text and Figures
Supplementary Fig.1 and Supplementary Methods (PDF 297 kb)
Rights and permissions
About this article
Cite this article
Bellinger, A., Reiken, S., Carlson, C. et al. Hypernitrosylated ryanodine receptor calcium release channels are leaky in dystrophic muscle. Nat Med 15, 325–330 (2009). https://doi.org/10.1038/nm.1916
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.1916
This article is cited by
-
Rapid restitution of contractile dysfunction by synthetic copolymers in dystrophin-deficient single live skeletal muscle fibers
Skeletal Muscle (2023)
-
Function of a mutant ryanodine receptor (T4709M) linked to congenital myopathy
Scientific Reports (2023)
-
Cell Therapy Strategies on Duchenne Muscular Dystrophy: A Systematic Review of Clinical Applications
Stem Cell Reviews and Reports (2023)
-
The prevention of home-cage grid climbing affects muscle strength in mice
Scientific Reports (2022)
-
The role of the dystrophin glycoprotein complex in muscle cell mechanotransduction
Communications Biology (2022)