Abstract
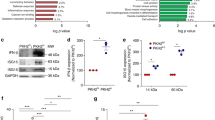
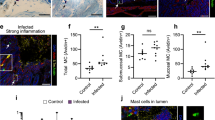
Sepsis remains a global clinical problem. By using the mouse cecal ligation and puncture model of sepsis, here we identify an important aspect of mast cell (MC)-dependent, innate immune defenses against Gram-negative bacteria by demonstrating that MC protease activity is regulated by interleukin-15 (IL-15). Mouse MCs express both constitutive and lipopolysaccharide-inducible IL-15 and store it intracellularly. Deletion of Il15 in mice markedly increases chymase activities, leading to greater MC bactericidal responses, increased processing and activation of neutrophil-recruiting chemokines, and significantly higher survival rates of mice after septic peritonitis. By showing that intracellular IL-15 acts as a specific negative transcriptional regulator of a mouse MC chymase (mast cell protease-2), we provide evidence that defined MC protease activity is transcriptionally regulated by an intracellularly retained cytokine. Our results identify an unexpected breach in MC-dependent innate immune defenses against sepsis and suggest that inhibiting intracellular IL-15 in MCs may improve survival from sepsis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Riedemann, N.C., Guo, R.F. & Ward, P.A. Novel strategies for the treatment of sepsis. Nat. Med. 9, 517–524 (2003).
Cohen, J. The immunopathogenesis of sepsis. Nature 420, 885–891 (2002).
Galli, S.J. et al. Mast cells as 'tunable' effector and immunoregulatory cells: recent advances. Annu. Rev. Immunol. 23, 749–786 (2005).
Marshall, J.S. Mast-cell responses to pathogens. Nat. Rev. Immunol. 4, 787–799 (2004).
Echtenacher, B., Männel, D.N. & Hültner, L. Critical protective role of mast cells in a model of acute septic peritonitis. Nature 381, 75–77 (1996).
Malaviya, R., Ikeda, T., Ross, E. & Abraham, N. Mast cell modulation of neutrophil influx and bacterial clearance at sites of infection through TNFα. Nature 381, 77–80 (1996).
Maurer, M. et al. The c-kit ligand, stem cell factor, can enhance innate immunity through effects on mast cells. J. Exp. Med. 188, 2343–2348 (1998).
Bone-Larson, C.L. et al. Novel protective effects of stem cell factor in a murine model of acute septic peritonitis. Dependence on MCP-1. Am. J. Pathol. 157, 1177–1186 (2000).
Feger, F., Varadaradjalou, S., Gao, Z., Abraham, S.N. & Arock, M. The role of mast cells in host defense and their subversion by bacterial pathogens. Trends Immunol. 23, 151–158 (2002).
Huang, C., Sali, A. & Stevens, R.L. Regulation and function of mast cell proteases in inflammation. J. Clin. Immunol. 18, 169–183 (1998).
Gurish, M.F. et al. Differential expression of secretory granule proteases in mouse mast cells exposed to interleukin 3 and c-kit ligand. J. Exp. Med. 175, 1003–1012 (1992).
Miller, H.R., Wright, S.H., Knight, P.A. & Thornton, E.M. A novel function for transforming growth factor-β1: upregulation of the expression and the IgE-independent extracellular release of a mucosal mast cell granule-specific β-chymase, mouse mast cell protease-1. Blood 93, 3473–3486 (1999).
Ghildyal, N., McNeil, H.P., Gurish, M.F., Austen, K.F. & Stevens, R.L. Transcriptional regulation of the mucosal mast cell-specific protease gene, MMCP-2, by interleukin 10 and interleukin 3. J. Biol. Chem. 267, 8473–8477 (1992).
Mizutani, H., Schechter, N., Lazarus, G., Black, R.A. & Kupper, T.S. Rapid and specific conversion of precursor interleukin 1β (IL-1β) to an active IL-1 species by human mast cell chymase. J. Exp. Med. 174, 821–825 (1991).
Zhao, W., Oskeritzian, C.A., Pozez, A.L. & Schwartz, L.B. Cytokine production by skin-derived mast cells: endogenous proteases are responsible for degradation of cytokines. J. Immunol. 175, 2635–2642 (2005).
Bulfone-Paus, S., Bulanova, E., Budagian, V. & Paus, R. The interleukin-15/interleukin-15 receptor system as a model for juxtacrine and reverse signaling. BioEssays 28, 362–377 (2006).
Fehniger, T.A. & Caligiuri, M.A. Interleukin-15: biology and relevance to human disease. Blood 97, 14–32 (2001).
Budagian, V., Bulanova, E., Paus, R. & Bulfone-Paus, S. IL-15/IL-15 receptor biology: a guided tour through an expanding universe. Cytokine Growth Factor Rev. 17, 259–280 (2006).
Hiromatsu, T. et al. Overexpression of interleukin-15 protects against Escherichia coli–induced shock accompanied by inhibition of tumor necrosis factor-α–induced apoptosis. J. Infect. Dis. 187, 1442–1451 (2003).
Colucci, F., Caligiuri, M.A. & Di Santo, J.P. What does it take to make a natural killer? Nat. Rev. Immunol. 3, 413–425 (2003).
Bulfone-Paus, S. et al. Interleukin-15 protects from lethal apoptosis in vivo. Nat. Med. 3, 1124–1128 (1997).
Bulanova, E. et al. Mast cells express novel functional IL-15 receptor α isoforms. J. Immunol. 170, 5045–5055 (2003).
Buras, J.A., Holzmann, B. & Sitkovsky, M. Animal models of sepsis: setting the stage. Nat. Rev. Drug Discov. 4, 854–865 (2005).
Malaviya, R. & Abraham, S.N. Role of mast cell leukotrienes in neutrophil recruitment and bacterial clearance in infectious peritonitis. J. Leukoc. Biol. 67, 841–846 (2000).
Dvorak, A.M. Subcellular localization of the cytokines, basic fibroblast growth factor and tumor necrosis factor-α in mast cells. Chem. Immunol. Allergy 85, 72–88 (2005).
Knight, P.A., Wright, S.H., Thornton, E.M., Brown, J. & Miller, H.R.P. Expression, function and regulation of mast cell granule chymases during mucosal allergic responses. In: Mast Cells and Basophils (eds. Marone G., Lichtenstein L.M. & Galli S.J.) 257–273 (Academic, San Diego, 2000).
Schiemann, F. et al. Mast cells and neutrophils proteolytically activate chemokine precursor CTAP-III and are subject to counter-regulation by PF-4 through inhibition of chymase and cathepsin G. Blood 107, 2234–2242 (2006).
Maurer, M. et al. Mast cells promote homeostasis by limiting endothelin-1–induced toxicity. Nature 432, 512–516 (2004).
Calandra, T. et al. Protection from septic shock by neutralization of macrophage migration inhibitory factor. Nat. Med. 6, 164–170 (2000).
Moreno, S.E. et al. IL-12, but not IL-18, is critical to neutrophil activation and resistance to polymicrobial sepsis induced by cecal ligation and puncture. J. Immunol. 177, 3218–3224 (2006).
Wirtz, S. et al. Protection from lethal septic peritonitis by neutralizing the biological function of interleukin 27. J. Exp. Med. 203, 1875–1881 (2006).
Kandere-Grzybowska, K. et al. IL-1 induces vesicular secretion of IL-6 without degranulation from human mast cells. J. Immunol. 171, 4830–4836 (2003).
Cao, J. et al. Human mast cells express corticotropin-releasing hormone (CRH) receptors and CRH leads to selective secretion of vascular endothelial growth factor. J. Immunol. 174, 7665–7675 (2005).
Strik, M.C. et al. Intracellular serpin SERPINB6 (PI6) is abundantly expressed by human mast cells and forms complexes with β-tryptase monomers. Blood 103, 2710–2717 (2004).
Feyerabend, T.B. et al. Loss of histochemical identity in mast cells lacking carboxypeptidase A. Mol. Cell. Biol. 25, 6199–6210 (2005).
Ilangumaran, S., Finan, D., Raine, J. & Rottapel, R. Suppressor of cytokine signaling 1 regulates an endogenous inhibitor of a mast cell protease. J. Biol. Chem. 278, 41871–41880 (2003).
Wilusz, C.J., Wormington, M. & Peltz, S.W. The cap-to-tail guide to mRNA turnover. Nat. Rev. Mol. Cell Biol. 2, 237–246 (2001).
Xia, Z., Ghildyal, N., Austen, K.F. & Stevens, R.L. Post-transcriptional regulation of chymase expression in mast cells. J. Biol. Chem. 271, 8747–8753 (1996).
de Garavilla, L. et al. A novel, potent dual inhibitor of the leukocyte proteases cathepsin G and chymase: molecular mechanisms and anti-inflammatory activity in vivo. J. Biol. Chem. 280, 18001–18007 (2005).
Lindstedt, K.A. et al. Activation of paracrine TGF-β1 signaling upon stimulation and degranulation of rat serosal mast cells: a novel function for chymase. FASEB J. 15, 1377–1388 (2001).
Nabah, Y.N. et al. Angiotensin II induces neutrophil accumulation in vivo through generation and release of CXC chemokines. Circulation 110, 3581–3586 (2004).
Kunori, Y. et al. Rodent α-chymases are elastase-like proteases. Eur. J. Biochem. 269, 5921–5930 (2002).
Solivan, S., Selwood, T., Wang, Z.M. & Schechter, N.M. Evidence for diversity of substrate specificity among members of the chymase family of serine proteases. FEBS Lett. 512, 133–138 (2002).
Di Nardo, A., Vitiello, A. & Gallo, R.L. Cutting edge: mast cell antimicrobial activity is mediated by expression of cathelicidin antimicrobial peptide. J. Immunol. 170, 2274–2278 (2003).
Yang, D. et al. LL-37, the neutrophil granule- and epithelial cell-derived cathelicidin, utilizes formyl peptide receptor-like 1 (FPRL1) as a receptor to chemoattract human peripheral blood neutrophils, monocytes, and T cells. J. Exp. Med. 192, 1069–1074 (2000).
Lodolce, J.P. et al. IL-15 receptor maintains lymphoid homeostasis by supporting lymphocyte homing and proliferation. Immunity 9, 669–676 (1998).
Kennedy, M.K. et al. Reversible defects in natural killer and memory CD8 T cell lineages in interleukin 15–deficient mice. J. Exp. Med. 191, 771–780 (2000).
Pasparakis, M., Alexopoulou, L., Episkopou, V. & Kollias, G. Immune and inflammatory responses in TNF α-deficient mice: a critical requirement for TNFα in the formation of primary B cell follicles, follicular dendritic cell networks and germinal centers, and in the maturation of the humoral immune response. J. Exp. Med. 184, 1397–1411 (1996).
Orinska, Z. et al. TLR3-induced activation of mast cells modulates CD8+ T-cell recruitment. Blood 106, 978–987 (2005).
Acknowledgements
We thank K. Streeck, D. Benner, S. Dinges and A. Bolch for technical support; C. Hölscher for IL-27 measurement; J. Urcioli for proofreading the manuscript; and J. Fingerle for advice. E. coli strain 2131 was a gift from O. Sperling and T.K. Lindhorst, Institute for Organic Chemistry, University of Kiel. This work was funded, in part, by grants from the Deutsche Forschungsgemeinschaft (DFG) to S.B.P. (SFB/TR22, A14), E.B. (SFB/TR22, A11), and M. Maurer (MA 1909/4-1/2). This work benefited from the European Union Network of Excellence Global Allergy and Asthma European Network (GA2LEN).
Author information
Authors and Affiliations
Contributions
Z.O. conducted the in vitro studies. M. Maurer and M. Metz conducted the in vivo experiments. F.M. conducted MCP-related experiments (expression, promoter regulation). N.N. and J.S. conducted the microbicidal assays. F.S. and E. Brandt performed and evaluated CTAP-III processing experiments. V.B. contributed to RT-PCR analysis. J. G.-M. and E. Bulanova conducted confocal staining experiments. Z.O., M. Maurer, R.P. and S.B.-P. wrote the manuscript. Z.O. and S.B.-P. supervised the project.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–6, Supplementary Table 1, Supplementary Methods (PDF 2402 kb)
Rights and permissions
About this article
Cite this article
Orinska, Z., Maurer, M., Mirghomizadeh, F. et al. IL-15 constrains mast cell–dependent antibacterial defenses by suppressing chymase activities. Nat Med 13, 927–934 (2007). https://doi.org/10.1038/nm1615
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm1615
This article is cited by
-
Mast cells form antibody-dependent degranulatory synapse for dedicated secretion and defence
Nature Communications (2015)
-
IL-15 functions as a danger signal to regulate tissue-resident T cells and tissue destruction
Nature Reviews Immunology (2015)
-
Potential effector and immunoregulatory functions of mast cells in mucosal immunity
Mucosal Immunology (2015)
-
Beneficial metabolic activities of inflammatory cytokine interleukin 15 in obesity and type 2 diabetes
Frontiers of Medicine (2015)
-
Insights into potential pathogenesis mechanisms associated with Campylobacter jejuni-induced abortion in ewes
BMC Veterinary Research (2014)