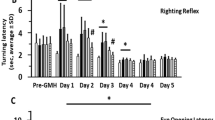
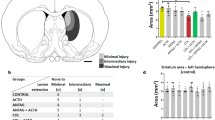
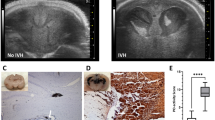
Abstract
The germinal matrix of premature infants is selectively vulnerable to hemorrhage within the first 48 h of life. To assess the role of vascular immaturity in germinal matrix hemorrhage (GMH), we evaluated germinal matrix angiogenesis in human fetuses and premature infants, as well as in premature rabbit pups, and noted active vessel remodeling in all three. Vascular endothelial growth factor (VEGF), angiopoietin-2 and endothelial cell proliferation were present at consistently higher levels in the germinal matrix relative to the white matter anlagen and cortical mantle. On that basis, we asked whether prenatal treatment with either of two angiogenic inhibitors, the COX-2 inhibitor celecoxib, or the VEGFR2 inhibitor ZD6474, could suppress the incidence of GMH in premature rabbit pups. Celecoxib treatment decreased angiopoietin-2 and VEGF levels as well as germinal matrix endothelial proliferation. Furthermore, treatment with celecoxib or ZD6474 substantially decreased the incidence of GMH. Thus, by suppressing germinal matrix angiogenesis, prenatal celecoxib or ZD6474 treatment may be able to reduce both the incidence and severity of GMH in susceptible premature infants.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Heuchan, A.M., Evans, N., Henderson Smart, D.J. & Simpson, J.M. Perinatal risk factors for major intraventricular haemorrhage in the Australian and New Zealand Neonatal Network, 1995–97. Arch. Dis. Child. Fetal Neonatal Ed. 86, F86–F90 (2002).
The Vermont-Oxford Trials Network. Very low birth weight outcomes for 1990. Investigators of the Vermont-Oxford Trial Network Database Project. Pediatrics 91, 540–545 (1993).
Ballabh, P., Braun, A. & Nedergaard, M. The blood-brain barrier: an overview: structure, regulation, and clinical implications. Neurobiol. Dis. 16, 1–13 (2004).
Du Plessis, A.J. & Volpe, J.J. Perinatal brain injury in the preterm and term newborn. Curr. Opin. Neurol. 15, 151–157 (2002).
Ballabh, P., Braun, A. & Nedergaard, M. Anatomic analysis of blood vessels in germinal matrix, cerebral cortex, and white matter in developing infants. Pediatr. Res. 56, 117–124 (2004).
Vates, G.E., Hashimoto, T., Young, W.L. & Lawton, M.T. Angiogenesis in the brain during development: the effects of vascular endothelial growth factor and angiopoietin-2 in an animal model. J. Neurosurg. 103, 136–145 (2005).
Wong, C.G., Rich, K.A., Liaw, L.H., Hsu, H.T. & Berns, M.W. Intravitreal VEGF and bFGF produce florid retinal neovascularization and hemorrhage in the rabbit. Curr. Eye Res. 22, 140–147 (2001).
Ferrara, N., Gerber, H.P. & LeCouter, J. The biology of VEGF and its receptors. Nat. Med. 9, 669–676 (2003).
Carmeliet, P. Angiogenesis in health and disease. Nat. Med. 9, 653–660 (2003).
Zhu, Y. et al. Angiopoietin-2 facilitates vascular endothelial growth factor-induced angiogenesis in the mature mouse brain. Stroke 36, 1533–1537 (2005).
Yancopoulos, G.D. et al. Vascular-specific growth factors and blood vessel formation. Nature 407, 242–248 (2000).
Suri, C. et al. Requisite role of angiopoietin-1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell 87, 1171–1180 (1996).
Hammes, H.P. et al. Angiopoietin-2 causes pericyte dropout in the normal retina: evidence for involvement in diabetic retinopathy. Diabetes 53, 1104–1110 (2004).
Maisonpierre, P.C. et al. Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science 277, 55–60 (1997).
Scharpfenecker, M., Fiedler, U., Reiss, Y. & Augustin, H.G. The Tie-2 ligand angiopoietin-2 destabilizes quiescent endothelium through an internal autocrine loop mechanism. J. Cell Sci. 118, 771–780 (2005).
Hanahan, D. Signaling vascular morphogenesis and maintenance. Science 277, 48–50 (1997).
Jain, R.K. Molecular regulation of vessel maturation. Nat. Med. 9, 685–693 (2003).
Wang, D., Buchanan, F.G., Wang, H., Dey, S.K. & DuBois, R.N. Prostaglandin E2 enhances intestinal adenoma growth via activation of the Ras-mitogen-activated protein kinase cascade. Cancer Res. 65, 1822–1829 (2005).
Pichiule, P., Chavez, J.C. & LaManna, J.C. Hypoxic regulation of angiopoietin-2 expression in endothelial cells. J. Biol. Chem. 279, 12171–12180 (2004).
Wu, Y.L., Fu, S.L., Zhang, Y.P., Qiao, M.M. & Chen, Y. Cyclooxygenase-2 inhibitors suppress angiogenesis and growth of gastric cancer xenografts. Biomed. Pharmacother. 59 (suppl. 2), S289–S292 (2005).
Masferrer, J.L. et al. Antiangiogenic and antitumor activities of cyclooxygenase-2 inhibitors. Cancer Res. 60, 1306–1311 (2000).
Dembo, G., Park, S.B. & Kharasch, E.D. Central nervous system concentrations of cyclooxygenase-2 inhibitors in humans. Anesthesiology 102, 409–415 (2005).
Hartleroad, J.Y. et al. Effect of maternal administration of selective cyclooxygenase (COX)-2 inhibitors on renal size, growth factors, proteinases, and COX-2 secretion in the fetal rabbit. Biol. Neonate 87, 246–253 (2005).
Stika, C.S. et al. A prospective randomized safety trial of celecoxib for treatment of preterm labor. Am. J. Obstet. Gynecol. 187, 653–660 (2002).
Herbst, R.S. et al. Phase I study of recombinant human endostatin in patients with advanced solid tumors. J. Clin. Oncol. 20, 3792–3803 (2002).
Virgintino, D. et al. VEGF expression is developmentally regulated during human brain angiogenesis. Histochem. Cell Biol. 119, 227–232 (2003).
Mayer, H. et al. Vascular endothelial growth factor (VEGF-A) expression in human mesenchymal stem cells: autocrine and paracrine role on osteoblastic and endothelial differentiation. J. Cell. Biochem. 95, 827–839 (2005).
Lorenzo, A.V., Welch, K. & Conner, S. Spontaneous germinal matrix and intraventricular hemorrhage in prematurely born rabbits. J. Neurosurg. 56, 404–410 (1982).
Leahy, K.M. et al. Cyclooxygenase-2 inhibition by celecoxib reduces proliferation and induces apoptosis in angiogenic endothelial cells in vivo. Cancer Res. 62, 625–631 (2002).
Sanborn, R. & Blanke, C.D. Cyclooxygenase-2 inhibition in colorectal cancer: boom or bust? Semin. Oncol. 32, 69–75 (2005).
Schnurch, H. & Risau, W. Expression of tie-2, a member of a novel family of receptor tyrosine kinases, in the endothelial cell lineage. Development 119, 957–968 (1993).
Key, G. et al. New Ki-67-equivalent murine monoclonal antibodies (MIB 1–3) generated against bacterially expressed parts of the Ki-67 cDNA containing three 62 base pair repetitive elements encoding for the Ki-67 epitope. Lab. Invest. 68, 629–636 (1993).
Isobe, K. et al. Measurement of cerebral oxygenation in neonates after vaginal delivery and cesarean section using full-spectrum near infrared spectroscopy. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 132, 133–138 (2002).
Alvarez-Soria, M.A. et al. Long term NSAID treatment inhibits COX-2 synthesis in the knee synovial membrane of patients with osteoarthritis: differential proinflammatory cytokine profile between celecoxib and aceclofenac. Ann. Rheum. Dis. 65, 998–1005 (2006).
El-Rayes, B.F., Ali, S., Sarkar, F.H. & Philip, P.A. Cyclooxygenase-2-dependent and -independent effects of celecoxib in pancreatic cancer cell lines. Mol. Cancer Ther. 3, 1421–1426 (2004).
Conner, E.S., Lorenzo, A.V., Welch, K. & Dorval, B. The role of intracranial hypotension in neonatal intraventricular hemorrhage. J. Neurosurg. 58, 204–209 (1983).
Papile, L.A., Burstein, J., Burstein, R. & Koffler, H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J. Pediatr. 92, 529–534 (1978).
Muscara, M.N., Vergnolle, N., Lovren, F., Triggle, C.R., Elliott, S.N., Asfaha, S. & Wallace, J.L. Selective cyclo-oxygenase-2 inhibition with celecoxib elevates blood pressure and promotes leukocyte adherence. Br. J. Pharmacol. 129, 1423–1430 (2000).
Tommiska, V. et al. A national short-term follow-up study of extremely low birth weight infants born in Finland in 1996–1997. Pediatrics 107, E2 (2001).
Beverley, D.W., Chance, G.W. & Coates, C.F. Intraventricular haemorrhage–timing of occurrence and relationship to perinatal events. Br. J. Obstet. Gynaecol. 91, 1007–1013 (1984).
Tsiantos, A. et al. Intracranial hemorrhage in the prematurely born infant. Timing of clots and evaluation of clinical signs and symptoms. J. Pediatr. 85, 854–859 (1974).
Sawaoka, H. et al. Cyclooxygenase inhibitors suppress angiogenesis and reduce tumor growth in vivo. Lab. Invest. 79, 1469–1477 (1999).
Amrite, A.C., Ayalasomayajula, S.P., Cheruvu, N.P. & Kompella, U.B. Single periocular injection of celecoxib-PLGA microparticles inhibits diabetes-induced elevations in retinal PGE2, VEGF, and vascular leakage. Invest. Ophthalmol. Vis. Sci. 47, 1149–1160 (2006).
Fukuda, R., Kelly, B. & Semenza, G.L. Vascular endothelial growth factor gene expression in colon cancer cells exposed to prostaglandin E2 is mediated by hypoxia-inducible factor 1. Cancer Res. 63, 2330–2334 (2003).
Lukiw, W.J. et al. Coordinate activation of HIF-1 and NF-kappaB DNA binding and COX-2 and VEGF expression in retinal cells by hypoxia. Invest. Ophthalmol. Vis. Sci. 44, 4163–4170 (2003).
Sakai, M. et al. Evaluation of the tocolytic effect of a selective cyclooxygenase-2 inhibitor in a mouse model of lipopolysaccharide-induced preterm delivery. Mol. Hum. Reprod. 7, 595–602 (2001).
The Boulder Committee. Embryonic vertebrate central nervous system: revised terminology. Anat. Rec. 28, 3019–3024 (1970).
Ballabh, P., Hu, F., Kumarasiri, M., Braun, A. & Nedergaard, M. Development of tight junction molecules in blood vessels of germinal matrix, cerebral cortex, and white matter. Pediatr. Res. 58, 791–798 (2005).
Acknowledgements
We thank J. Etlinger for critical evaluation of the manuscript and J. Abrahams for technical and editorial assistance. We thank T. Takano and X. Han for assistance with confocal imaging. Supported by the US National Institutes of Health/National Institute of Neurological Disorders and Stroke grants NS050586 to P.B., R01NS29813 to S.G., and Ro130007 and the Philip Morris Organization to M.N.
Author information
Authors and Affiliations
Contributions
P.B., in whose laboratory this study was performed, planned and supervised experiments, performed confocal microscopy, data analysis and co-wrote the manuscript; H.X. and F.H. performed the animal experiments, immunostaining and western blot analysis; A.B. chose and contributed samples and assisted in editing the manuscript; A.R., K.S., Z.U. and A.C. contributed to laser dissection of brain sections, and to PCR on human and rabbit brains, and also performed the related data analysis; N.L. performed intracranial pressure and cerebral blood flow monitoring of the rabbit pups; S.G. and M.N. assisted in experimental design and planning, and co-wrote the manuscript; M.N. provided direct support for the study.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
VEGF expression in blood vessels and glial cells of human GM, cortex and WM. (PDF 969 kb)
Supplementary Fig. 2
VEGFR1 expression comparable among cortex, WM and GM in human and rabbit subjects. (PDF 113 kb)
Supplementary Fig. 3
VEGFR2 protein and mRNA expression similar in human and rabbit neonates. (PDF 143 kb)
Supplementary Fig. 4
Tie 2 expression comparable among cortex, WM and GM in humans and rabbits. (PDF 189 kb)
Supplementary Fig. 5
Greater expression of ANGPT-1 protein in fetuses than premature infants. (PDF 355 kb)
Supplementary Fig. 6
Intracranial cerebral pressure, cerebral blood flow, and mean arterial blood pressure on glycerol treatment in celecoxib treated as well as untreated pups. (PDF 55 kb)
Supplementary Fig. 7
Glycerol does not affect angiogenesis and angiogenic factors. (PDF 23 kb)
Supplementary Fig. 8
ZD6474 downregulates VEGFR2 and ERK1/2 phosphorylation. (PDF 170 kb)
Rights and permissions
About this article
Cite this article
Ballabh, P., Xu, H., Hu, F. et al. Angiogenic inhibition reduces germinal matrix hemorrhage. Nat Med 13, 477–485 (2007). https://doi.org/10.1038/nm1558
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm1558
This article is cited by
-
Evaluation of recombinant human IGF-1/IGFBP-3 on intraventricular hemorrhage prevention and survival in the preterm rabbit pup model
Scientific Reports (2023)
-
The Role of Oxidative Stress in the Progression of Secondary Brain Injury Following Germinal Matrix Hemorrhage
Translational Stroke Research (2023)
-
Mechanisms of neuroinflammation in hydrocephalus after intraventricular hemorrhage: a review
Fluids and Barriers of the CNS (2022)
-
Pharmacological neuroprotection and clinical trials of novel therapies for neonatal peri-intraventricular hemorrhage: a comprehensive review
Acta Neurologica Belgica (2022)
-
Mortality and neurological outcomes in extremely and very preterm infants born to mothers with hypertensive disorders of pregnancy
Scientific Reports (2021)