Abstract
We found that the proteome of apoptotic T cells includes prominent fragments of cellular proteins generated by caspases and that a high proportion of distinct T cell epitopes in these fragments is recognized by CD8+ T cells during HIV infection. The frequencies of effector CD8+ T cells that are specific for apoptosis-dependent epitopes correlate with the frequency of circulating apoptotic CD4+ T cells in HIV-1–infected individuals. We propose that these self-reactive effector CD8+ T cells may contribute to the systemic immune activation during chronic HIV infection. The caspase-dependent cleavage of proteins associated with apoptotic cells has a key role in the induction of self-reactive CD8+ T cell responses, as the caspase-cleaved fragments are efficiently targeted to the processing machinery and are cross-presented by dendritic cells. These findings demonstrate a previously undescribed role for caspases in immunopathology.
Similar content being viewed by others
Main
In apoptotic cells, the activation of caspases leads to significant alterations of the proteome1. Apoptosis represents a key step in the homeostasis of the immune system because it is involved in the purging of autoreactive lymphocytes during central and peripheral tolerance, as well as in the termination of effector immune responses2. On the other hand, apoptotic cells are considered to be an important source of self antigens that elicit autoantibodies, which are frequently associated with systemic autoimmune diseases3,4. Furthermore, the phagocytosis of apoptotic cells by dendritic cells (DCs) leads to the processing of apoptotic cell–associated (apoptotic) antigens and the cross-presentation of the resulting peptides on major histocompatibility complex class I molecules5. This phenomenon seems to be crucial for inducing either cross-priming or cross-tolerance of CD8+ T cells, depending on the presence or absence of various infectious or danger signals that influence the switch from tolerogenic immature DCs (iDCs) to mature DCs (mDCs) with high stimulatory and migratory capacities6,7,8,9,10,11,12,13.
Infection with HIV type 1 leads to a profound depletion of CD4+ T cells that largely depends on activation-induced cell death rather than infection14,15,16,17. Indeed, the progression of HIV infection correlates with chronic hyperreactivity of T and B cells, which results in a continuous turnover of apoptotic cells14,15,16,17. This systemic immune activation seems to facilitate HIV replication, the emergence of new viral variants and, in the long-term, extensive T cell dysfunction that ultimately leads to AIDS14,17. Chronic T cell activation is only minimally attributable to HIV-specific T cells, as only a small proportion of activated T cells is specific for the virus14,15,17. Its origin is not clear and has been ascribed to various causes including bystander activation17, homeostatic proliferation14, T cell responses to a variety of self antigens or gut microorganisms14,18,19 and progressive loss of regulatory T cells17,20. If we can define the causes of chronic immune activation, it might be possible to design innovative strategies for the manipulation of immune responses in immunopathology.
Here we show that, first, apoptotic T cells generate protein fragments that have antigenic properties with respect to human CD8+ T cells; second, these autoreactive CD8+ T cell responses correlate with the proportion of apoptotic CD4+ T cells in individuals with HIV infection in vivo; and third, the caspase-mediated cleavage of cellular components during apoptosis facilitates antigen processing and cross-presentation by DCs.
Results
Proteomic analysis of apoptotic or live T cells
Six independent CD95+CD8+ T cell clones underwent apoptosis as a result of treatment with a monoclonal antibody (mAb) to CD95. The apoptotic and the corresponding live (control) cells were promptly lysed and compared using subtractive analysis by two-dimensional electrophoresis (2DE) to identify modified proteins. 2DE represents the system of choice for measuring the changes in protein expression (abundance) or structure (differences in molecular weight or isoelectric point) that occur during apoptosis1. Approximately 500 spots were resolved and detected by Coomassie blue staining (Fig. 1a,b). Of these spots, 13 were exclusively detected in protein patterns of apoptotic cells, 23 were found to be at least half as abundant in apoptotic cells as in control cell clones, and 6 were at least twice as abundant in apoptotic cells as in control cells (Fig. 1a,b and Table 1). Analysis of these 42 spots with matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF-MS) showed that 25 were identified as known proteins by peptide mass fingerprinting (Table 1). Most of the spots found only in apoptotic cells corresponded to fragments derived from cytoskeletal proteins (nonmuscle myosin, cytoplasmic actin, vimentin and lamin B1), proteins involved in the regulation of the cytoskeleton (Rho guanine nucleotide dissociation inhibitor-2 (Rho GDPI2) and T complex protein 1β subunit), or nuclear lamina and matrix proteins (heterogeneous nuclear ribonucleoprotein K (hnRNPK)) (Table 1). The whole-form versions of most of the proteins were found to decrease during apoptosis, indicating that the related fragments were products of apoptosis-associated proteolysis1 (Table 1 and Fig. 1a,b). In contrast, a number of proteins were either downregulated without the appearance of their cleavage products (phosphoglycerate mutase-1, ubiquitous tropomodulin, upstream of N-ras interacting protein, annexin V, coronin-like protein p57 and T complex protein-1β subunit) or upregulated (60S acidic ribosomal protein P2 and proteasome subunit-α type 1) in apoptotic cells with respect to control cells (Table 1 and Fig. 1a,b). Several of these proteins have been reported to be targets of autoantibodies, thus supporting the interaction between apoptosis and autoimmunity3,4,21.
(a,b) these subjects Coomassie brilliant blue–stained 2DE gel of total cell extract proteins from one representative of six antibody to CD95–treated CD8+ T cell clones (apoptotic cells, ACs); (a) or from one representative of the corresponding live clones (control cells, CCs); (b). Hexagons denote spots whose relative abundance is at least twice as much in one sample as in the other. These spots were excised, in-gel digested with trypsin, and analyzed by MALDI-TOF-MS (numbers correspond to those in Table 1). MW, molecular weight. (c) Percentage of HLA-A2–binding self peptides (derived from 2DE-identified proteins) recognized by IFN-γ+efCD8+ T cells (detected by ELISPOT assay) from subjects with HIV. Because of the limited number of PBMCs obtained from these subjects, freshly isolated CD8+ T cells were tested against 91 peptides, randomly selected from the 251 shown in Supplementary Table 1a–h (indicated with peptide identity (ID) numbers in Supplementary Figure 1). The bars represent the percentage of self-peptides recognized by efCD8+ T cells whose spot-forming cell (SFC) values were twofold higher than the background; the yellow-plus-red fractions of the bars represent those with SFC values threefold higher; the red fractions alone represent those with SFC values fourfold higher. The responses to the individual peptides are shown in Supplementary Figure 1. (d) Percentage of 251 HLA-A2–binding self peptides recognized by IFN-γ+efCD8+ T cells from healthy donors whose SFC values were twofold higher than background.
Effector CD8+ T cells specific to cell-associated proteins
To verify the immunogenicity of the proteins that were identified with 2DE (Table 1), we prepared a wide array of synthetic HLA-A2–binding peptides derived from them22 (Supplementary Table 1a–h online). Then, we tested freshly isolated effector (ef)CD8+ T cells from either HLA-A2+ individuals with chronic HIV infection (Supplementary Table 2 online) or healthy donors for the capacity to form interferon γ (IFN-γ) spots within 4–6 h of contact with the single peptides in an enzyme-linked immunospot (ELISPOT) assay. We found a large repertoire of IFN-γ+efCD8+ T cells specific to multiple self peptides in most of the 42 infected subjects tested (Fig. 1c). However, despite the multispecificity of these CD8+ T cells, they appeared at very low frequencies (Supplementary Fig. 1 online). By contrast, antiviral IFN-γ+efCD8+ T cells appeared at high frequencies against a limited number of immunodominant viral epitopes (Gag77–85, Env731–739, Nef158–167) in the same individuals (Supplementary Fig. 2 and Supplementary Table 3 online). These data are consistent with evidence that T cells specific for immunodominant self determinants are usually deleted during thymic selection, whereas those that are positively selected are generally specific for multiple subdominant or cryptic self epitopes (that is, peptides that are poorly processed and presented in the thymus) and can provoke autoimmunity23.
None of the 23 HLA-A2+ healthy donors tested showed significant effector responses against any of these peptides ex vivo (Fig. 1d), supporting the possibility that the IFN-γ+efCD8+ T cells in HIV-infected individuals were primed in vivo. However, 8 of 23 healthy donors showed numerous resting (re)CD8+ T cells that formed IFN-γ spots after two rounds of peptide stimulation in vitro (Supplementary Fig. 3 online). Thus, these self-reactive CD8+ T cells seem to be silent or undetectable in the normal repertoire2,12 but differentiate in effector cells during severe inflammatory processes (for example, chronic HIV infection), according to the model of 'danger' as a crucial signal in breaking tolerance10,11,12,13. The HLA restriction of CD8+ T cell responses was highlighted by the findings that they were inhibited by an appropriate mAb to MHC class I and that 14 HLA-A2− individuals (six healthy donors and eight HIV-infected subjects) tested did not respond to HLA-A2-binding peptides (data not shown). Notably, HIV-infected subjects also responded to peptides derived from two proteins (60S acidic ribosomal protein P2 and proteasome subunit-α type 1) that were found to be upregulated, but not fragmented, in our proteomic analysis of apoptotic T cells (Fig. 1a, Table 1 and Supplementary Table 1h online). However, because both of these proteins have been found to be cleaved by caspases in human apoptotic Jurkat or lymphoma cell lines1,24,25, we hypothesize that their related fragments were not sufficiently abundant in apoptotic T cells to be detectable by 2DE analysis.
Enumeration of CD8+ T cells by HLA-A2 pentamers in vivo
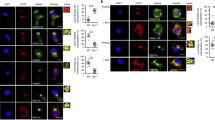
To visualize specific CD8+ T cells in vivo, we enumerated them directly in the peripheral blood of selected HIV-infected individuals by using pentamers of HLA-A*0201 molecules complexed to relevant self peptides. All pentamer+ cells were confined in the CD8+ subset, as shown by dot-plot analyses (Fig. 2a–c and Supplementary Fig. 4 online). The pentamer values were <0.02% (mean ± s.d. = 0.0153% ± 0.0159% for all pentamers analyzed) in 10 HLA-A2+ healthy individuals; thus 0.02% was considered as the sensitivity level of the assay. Virtually all pentamer+ cells showed downregulation of T cell receptors in response to the relevant peptide, further supporting the specificity of pentamer detection (Fig. 2d). Notably, the total frequencies of CD8+pentamer+ cells were significantly higher than those of IFN-γ+efCD8+ T cells detected by ELISPOT assay (IFN-γ spots; Fig. 2e). However, the frequencies of CD8+pentamer+ cells producing IFN-γ in response to peptides (Fig. 2a–c and Supplementary Fig. 4) tended to be related to those of IFN-γ spots (Fig. 2e). These data support the presence of these CD8+ T cells in HIV-infected individuals in vivo and indicate that only a minority of them shows effector function ex vivo in terms of IFN-γ production. However, the effector phenotype was substantiated by both perforin and granzyme expression in a considerable proportion of fresh pentamer+CD8+ cells from all infected subjects studied (Fig. 2f), which suggests that these cells have a potential role in immunopathology. Further studies are in progress to determine whether some of these cells show alternative phenotypes (for example, central memory, IL-10-production, Th17 or exhausted phenotypes). Of note, prompt IFN-γ production ex vivo by pentamer+CD8+ T cells from subjects with HIV (but not from healthy donors; data not shown) was elicited by DCs that had been pulsed with apoptotic cells derived from CD95+ T cell clones (Fig. 2g). These data indicate that IFN-γ+efCD8+ T cells recognizing naturally processed epitopes derived from apoptotic proteins are present in the peripheral repertoire of HIV-infected individuals in vivo.
(a–c) Flow cytometry analyses of PBMCs from three HIV-infected subjects (for analyses of five other subjects, see Supplementary Fig. 4) double-stained with pentamers expressing the indicated peptides of nonmuscle myosin (MYH9), vimentin (VIME) or actin (ACTB) and mAb to CD8. The percentage of CD8+pentamer+ cells is indicated in each quadrant. Contour plot analyses are gated on CD8+pentamer+ cells that were or were not stimulated with the relevant peptides (pep.), and show percentage of IFN-γ+ cells. (d) Representative experiment in which PBMCs from the subject shown in c were stimulated with the indicated peptide and then double-stained with pentamer (Pent.) expressing the MYH9 peptide and mAb to CD8. Dot plots are gated on CD8+ cells and show percentage of pentamer+ cells. (e) Percentages of indicated total CD8+ pentamer+ T cells and those producing IFN-γ compared with values of CD8+ T cells producing IFN-γ in response to the same epitopes, as determined by ELISPOT assay. (f) Representative experiment in which PBMCs from one subject were stained with pentamers expressing the indicated peptides and with mAbs to CD8, perforin and granzyme B. Dot plots are gated on CD8+pentamer+ cells and show percentage of perforin+ granzyme B+ cells. (g) Dot plot analysis of PBMCs from one subject double-stained with pentamers and mAb to CD8. Contour plots are gated on CD8+pentamer+ cells stimulated with autologous iDCs that have been pulsed or not with apoptotic cloned T cells (ACs) and show percentage of IFN-γ+ cells.
Autoreactive CD8+ T cells and apoptosis are correlated in vivo
The percentage of apoptotic annexin-V+ CD4+ cells in subjects with HIV was directly correlated with the sum of IFN-γ spots promptly formed by efCD8+ T cells in response to each apoptosis-associated peptide (Fig. 3a). Thus, despite the low frequency of the single responses, the magnitude of the total responses might be relevant to sustaining chronic immune activation (Fig. 3). The percentage of apoptotic CD4+ T cells in the fresh peripheral blood mononuclear cells (PBMCs) was significantly higher in subjects with HIV than in 20 healthy donors tested (15.0% ± 10.8% versus 3.9% ± 3.9%; P < 0.0001). Moreover, the strength of the self-reactive responses was significantly and inversely correlated with the absolute number of peripheral live CD4+ T cells (Fig. 3c). In contrast, there was no correlation between either the percentages of apoptotic cells or the absolute number of live CD4+ T cells and the sum of IFN-γ spots promptly formed by efCD8+ T cells (Fig. 3b,d) in response to HIV-related epitopes (Supplementary Fig. 2 and Supplementary Table 3). In addition, the response strength significantly decreased in those individuals that responded to an appropriate antiviral therapy, but not in those that did not respond to the therapy, in relation to a decrease in both the percentage of circulating apoptotic CD4+ T cells and serum HIV RNA copies (Fig. 3e).
(a,b) Correlation between percentage of circulating apoptotic annexin+CD4+ T cells and sum of SFCs formed by efCD8+ T cells in response to apoptotic self epitopes in 42 HIV-infected individuals (a) or HIV epitopes in 22 individuals (b). NS, not significant. (c,d) Correlation between number of live CD4+ T cells and sum of SFCs formed by efCD8+ T cells in response to apoptotic self epitopes in 42 subjects (c) or HIV epitopes in 22 subjects (d). Each circle represents one subject. (e) Changes in frequencies of efCD8+ T cells responding to pools 1 or 2 of self peptides derived from proteins identified in the proteomic analysis of apoptotic T cells (mean of responses to the two pools), in the viral load (HIV-RNA copies), and in the percentage of circulating apoptotic annexin+CD4+ T cells, before and after antiretroviral therapy. In all conditions, results are expressed as SFCs in 1 × 106 CD8+ T cells. Each symbol represents one subject: open symbols represent responders to antiviral therapy, filled symbols represent non-responders.
Apoptosis-related fragmentation and cross-presentation
In functional experiments, CD8+ T cell clones that were specific for HLA-A2–restricted vimentin324–332, Rho GDPI2100–109, nonmuscle myosin1045–1053 or hRNPK193–202 (isolated from the peripheral blood of infected subjects or healthy donors; Fig. 4a) responded to iDCs that had been pulsed with apoptotic T cells expressing the protein fragments with the appropriate epitopes (Table 1) more than to those pulsed with control lysed cells expressing only higher amounts of the corresponding intact forms (Fig. 4b). Similarly, freshly isolated myeloid (my)DCs (Supplementary Fig. 5 online) more efficiently stimulated vimentin-specific CD8+ T cells when they cross-presented apoptotic cells (Supplementary Data 1, Supplementary Fig. 6a online). These data indicate that protein fragmentation during apoptosis improves the processing and cross-presentation of several apoptotic antigens by DCs. Notably, live DCs (both immature and mature) alone were unable to stimulate directly the various CD8+ T cell clones examined (Fig. 4b), even though DCs expressed the related intact proteins26, as confirmed by 2DE analysis (Supplementary Fig. 7a,b online). The slight response of CD8+ T cells to myDCs alone was probably due to the cross-presentation of the discrete proportion of myDCs undergoing apoptosis following the isolation procedures (Supplementary Data 1 and Supplementary Figs. 5c,d, 6a and 7c,d). Therefore, we speculate that live DCs, because of their lack of caspase-dependent protein cleavage, cannot prepare the proteasome substrates that are essential for endogenously generating sufficient amounts of those cellular epitopes that emerge during the cross-presentation of apoptotic cells.
(a) Responses of HLA-A2–restricted CD8+ T cell clones to vimentin324–332, Rho GDPI2100–109, hnRNK193–202 and myosin1045–1053 peptides (left to right) to the related peptides presented by HLA-A2+ (diamonds) or HLA-A2− (squares) DCs. Circles show response to iDCs that had been pulsed in the presence of lactacystin (L) with both peptide- and caspase inhibitor–treated apopotic cells. (b) Responses of the same clones to HLA-A2+ iDCs alone, mDCs alone or iDCs that had been previously pulsed (at a 1:10 ratio) with CCs, apoptotic cells ACs, ACs that had been previously treated with caspase-3 inhibitor (ACs + C3I), caspase-8 inhibitor (ACs + C8I) or the negative control (ACs + K), or ACs in the presence of L. (c) Responses of HLA-A2–restricted, NS31406–1415-specific CD8+ T cell clones to iDCs alone, iDCs pulsed with wild-type (WT), NS3-expressing (NS3+) control or apoptotic EBV-B cells (CCs or ACs), in the presence or absence of L. In some experiments, HLA-A2+ iDCs were pulsed with NS3+ CCs or NS3+ ACs that had been previously treated with both C3I and C8I (CIs). (b,c) Results represent the mean ± s.d. of three different experiments and are expressed as IFN-γ SFCs in 2 × 104 cloned CD8+ T cells.
Caspase-dependent fragmentation improves cross-presentation
Next, we showed that caspases have a role in preparing apoptotic cell substrates for subsequent processing and presentation by DCs. The treatment of apoptotic cells with selective inhibitors of caspase-3 or caspase-8 (C3I or C8I, but not negative control treatment) significantly blocked the cross-presentation of the relevant apoptotic antigens to the various specific CD8+ T cell clones tested (Fig. 4b). Treatment of apoptotic cells with caspase inhibitors markedly downregulated protein fragmentation as compared with apoptotic cells treated with a negative control (Supplementary Fig. 7e,f). Similar results were obtained with freshly isolated myDCs (Supplementary Fig. 6a). The treatment of DCs with the proteasome inhibitor lactacystin during pulsing with apoptotic cells strongly blocked cross-presentation (Fig. 4b). Conversely, apoptotic peptide presentation by DCs occurred efficiently even when these cells were pulsed with caspase-inhibitor–treated apoptotic cells in the presence of lactacystin, thus ruling out the possibility that apoptotic cells, caspase inhibitors or lactacystin could affect the stimulatory capacities of DCs (Fig. 4a). In addition, we excluded the possibility that caspase inhibitors might affect either apoptosis induction (Supplementary Fig. 8a online) or the ability of DCs to phagocytose apoptotic cells (Supplementary Fig. 8b,c). Control experiments also ruled out the possibility that cells treated with caspase inhibitors might affect the processing machinery in DCs. Indeed, apoptotic or control lysed cells that had been infected with vaccinia virus expressing the HCV nonstructural (NS)-3 antigen (NS3+ apoptotic or control cells) were efficiently cross-presented by DCs to NS31406–1415-specific CD8+ T cell clones27, irrespective of treatment with caspase inhibitors (Fig. 4c). Whether caspase-dependent cleavage is required for processing and cross-presentation of long-lived proteins (such as most of the fragmented proteins from apoptotic T cells (Table 1)) and not of short-lived proteins (such as cell-associated HCV-NS3)27,28, remains a relevant issue that requires further study. Moreover, the cross-presentation of NS3+ control cells (Fig. 4c) rules out the possibility that the inefficient cross-presentation of cell-associated antigens from control cells (Fig. 4b) was a result of the incapacity of iDCs to phagocytose them. Additional controls supported the specific role of caspases in the cross-presentation of cell-associated antigens (Supplementary Data 2, to Supplementary Fig. 6b).
Cross-presentation of cell-purified cytoskeletal proteins
We transferred 2DE gels containing control or apoptotic cell-derived spots onto nitrocellulose membranes and cut and eluted spots corresponding to either the whole (spot 2335–465) or fragmented vimentin sequences containing (spot 3196–426) or not containing (spot 13100–183 and spot 613–183) the relevant CD8+ T cell epitope324–332 (Fig. 1a and Table 1). The specificity of this system was highlighted by the finding that DCs could cross-present, in a proteasome-dependent fashion, both the whole and the fragmented vimentin forms expressing the relevant CD8 T cell epitope (Fig. 5a). The possibility that the cross-presentation of the 2DE gel–derived whole vimentin was caused by partial degradation related to the SDS-PAGE procedures was ruled out by the observation that DCs similarly cross-presented an intact (see the second and first lanes in Fig. 5b and c, respectively) form of recombinant (r)-vimentin1–465 (Fig. 5a). In addition, DCs cross-presented both the entire r-vimentin and its 20-kD fragment, obtained upon caspase cleavage in vitro (Fig. 5b) and containing the relevant CD8+ T cell epitope (Fig. 5a). Collectively, these data indicate that particular cell-associated antigens (such as long-lived proteins stably anchored to the cytoskeleton) normally do not access efficiently the cross-presentation pathway, unless they are tailored by caspases during apoptosis and then released from the cellular matrix to be delivered to the processing machinery.
(a) A HLA-A2–restricted vimentin324–332-specific CD8+ T cell clone was tested for its capacity to form IFN-γ spots in response to HLA-A2+ iDCs alone or pulsed with about 10 μg/ml of the indicated compounds. Wh. = whole; Fr. = fragmented; vim. = vimentin; r. = recombinant. Results represent the mean ± s.d. of six experiments and are expressed as SFCs in 2 × 104 cloned CD8+ T cells (** versus *: P < 0.05; ## versus #: not significant). The position numbers of protein sequences tested, their molecular weights, and the spot numbers corresponding to the proteins identified in the proteomic analysis (see Supplementary Fig. 1 and Table 1) are represented in parentheses. (b) Western immunoblot analysis of entire r-vimentin, treated or not with caspase-3 (C3) in vitro. The circled band represents the vimentin 20-kDa fragment separated by gel electrophoresis and used in cross-presentation assays. (c) Control immunoblot analyses of vimentin expressed by CCs or ACs treated or not with C3I, C8I or the caspase control (K). The preparations were then immunoblotted with the relevant guinea pig polyclonal antibody (recognizing entire and fragmented 40- and 20-kDa vimentin) and a secondary HRP-conjugated donkey antibody to guinea pig IgG Ab. Entire r-vimentin was used as positive control.
Discussion
Here we have defined a new role of caspases in facilitating the cross-presentation of apoptotic cell-associated antigens. In individuals with chronic HIV infection, apoptotic T cells seem to induce a wide repertoire of efCD8+ T cells that are specific to apoptotic epitopes in vivo. The magnitude of the efCD8+ T cell response directed against apoptotic cell–derived peptides correlated with the decline of circulating CD4+ T cells, suggesting that these self-reactive CD8+ T cells contribute to the immunopathology of HIV-1 infection. The presence of these CD8+ T cells in HIV-infected subjects with decreased CD4+ T cell help may be due to the capacity of DCs to be independently stimulated by a variety of signals (for example, toll-like receptor ligands, or inflammation danger)8,10,11,12,29, or even by apoptotic T cells expressing the CD40 ligand (CD40L)13.
Chronic HIV infection is associated with generalized hyperreactivity of T and B cells that is correlated with progressive immune system dysfunction and has been attributed to various causes14,15,16,17,18,19,20. We now propose an additional mechanism by which efCD8+ T cells that are specific to multiple apoptotic self epitopes may contribute to chronic immune activation in HIV infection. The observation that cross-presentation of apoptotic cells activates efCD8+ T cells in individuals with HIV ex vivo indicates that this mechanism might be operative in generating the variety of apoptotic antigen-specific CD8+ T cells found in these people, as well as in several experimental models5,10.
Cross-presentation of various apoptotic cell–associated antigens was strongly inhibited by blocking caspase-dependent cleavage of proteins in apoptotic cells. This, together with the finding that proteasome treatment of DCs inhibited cross-presentation of apoptotic antigens, indicates that a previously undescribed function of caspase-mediated cleavage during apoptosis is to provide proteasome substrates to DCs28,30,31 and, thus, to facilitate the processing and cross-presentation of apoptotic antigens. However, our model does not exclude the possibility that additional processing mechanisms might participate in the cross-presentation of caspase-cleaved proteins32,33,34. Nonetheless, our results indicate that caspase cleavage allows the release of (possibly long-lived35,36) cell-associated antigens from the cellular matrix and their subsequent delivery to the cross-presentation pathway. The generation of a antigenic repertoire in apoptotic cells by caspase-dependent cleavage might have an important role in inducing either cross-tolerance (in the steady state) or cross-priming (in pathological conditions) of self-reactive CD8+ T cells10,11,12,13,37. This process might contribute to the improved caspase-dependent immunogenicity observed in some autoimmune and tumor models13,38,39.
In conclusion, it is tempting to speculate, on the basis of our data, that the chronic immune activation that is commonly observed during chronic viral or autoimmune diseases13,14,17,18,19,20,38 results partly from a cycle whereby the cross-presentation of apoptotic T cells leads to the activation of a large repertoire of apoptotic antigen-specific T cells, which in turn undergo apoptosis after they have performed their effector functions, and so on. Depending on the amplification of this process, it might ultimately contribute to the irreversible impairment of the immune system, as in the case of HIV infection. These findings may have implications for tuning immune homeostasis in diseases affected by excessive T cell apoptosis. Conversely, the identification of caspase-cleaved proteins from apoptotic tumor cells might enable the improvement of anti-tumor immunotherapy in combination with efficient adjuvants, appropriate chemotherapy39, and even compounds that enhance cross-presentation by inhibiting phago-endosomal acidification27.
Methods
Healthy and HIV-infected subjects.
We studied 51 HIV-infected (Supplementary Table 2) and 23 healthy individuals (age, 25–50 years; 12 males, 11 females) according to the ethical guidelines of the 1975 Declaration of Helsinki and a priori approval by our Institutional Review Board. Individuals from these populations were selected for the expression of the HLA-A2 allele, as detected by flow cytometry using specific mAb (BB7.2, ATCC). None of the subjects with HIV was being treated with antiviral therapy at the start of the study. The number of PBMCs was enough to study CD8+ T cell responses against 91 apoptotic self peptides, which were randomly selected from the 251 shown in the Supplementary Table 1a–h, in 42 of the 51 infected subjects (Supplementary Fig. 1). The responses against a panel of HIV peptides were also studied in 22 of these 42 subjects (Supplementary Fig. 2). In the remaining nine HLA-A2+ HIV-infected individuals (indicated with an asterisk in Supplementary Table 2), the limited amount of PBMCs obtained allowed us to study only responses to two pools of apoptotic self peptides before and after four months of anti-retroviral therapy (two nucleoside reverse transcriptase inhibitors (zidovudine and lamivudine) and one protease inhibitor (lopinavir or ritonavir)) (Fig. 3e). The pools consisted of the following self peptides, indicated with the related protein acronym and peptide identity (ID) number, shown in Supplementary Table 1a–g: Pool 1: VIME 3002.0200, VIME 3002.0107, VIME 3002.0117, VIME 3002.0104, VIME 3002.0199, VIME 3002.0109, VIME 3002.0119, VIME 3002.0206, GDIS 3002.0135, GDIS 3002.0133; Pool 2: ACTB 3002.0130, ACTB 3002.0126, ACTB 3002.0131, ACTB 3002.0127, LAM 3002.098, LAM 3002.097, LAM 3002.099, LAM 3002.0196, LAM 3002.085, LAM 3002.0101.
Cell preparations.
We obtained PBMCs, highly purified CD8+ T cells, T cell clones, immature or mature monocyte-derived DCs, and fresh myeloid DCs as described previously13,40,41, and in Supplementary Methods online.
Apoptotic and control cell preparations.
We plated cloned CD8+CD95+ T cells (10–100 × 106) in a 24-well plate and incubated them for 1 h at 37 °C in the presence or absence of 14 μg/ml C3I (Z-DEVD-FMK), C8I (Z-IETD-FMK) or a negative caspase control (K, Z-FA-FMK) (all BD Biosciences Pharmingen) and then induced them to undergo Fas-mediated apoptosis as described13 (Supplementary Methods). Control cells were represented by live cloned T cells, which were promptly lysed by repeated freezing and thawing. We measured spontaneous apoptosis of CD4+ T cells in the peripheral blood of both HIV-infected and healthy individuals by annexin-V, PI (Sigma Aldrich) and phycoerythrin (PE)-Cy7–conjugated mAb to CD4 (Caltag) staining of fresh PBMCs before and after 18-h incubation in complete medium at 37 °C.
DC phagocytosis.
We evaluated the phagocytosis capacity of DCs as described42 and as reported in Supplementary Methods in detail.
Two-dimensional electrophoresis.
MALDI-TOF-MS analysis and database searching.
We excised each 2DE spot of interest and in-gel digested each one with trypsin as described43,44 and then analyzed them as described in Supplementary Methods.
Protein purification from two-dimensional electrophoresis gels.
We performed protein purification from 2DE gels as described45 and as reported in Supplementary Methods in detail.
Immunoblot of cellular vimentin.
We prepared different types of cell preparation, centrifuged them, prepared the pellets, quantified protein contents and separated them by SDS-PAGE as described46,47 (Supplementary Methods). We then transferred each gel to a nitrocellulose membrane (Schleicher & Schuell) and immunoblotted the proteins for vimentin as described in Supplementary Methods.
Caspase 3 digestion and SDS-PAGE of recombinant vimentin.
Human r-vimentin (Research Diagnostic) was digested by caspase-3 (C1224, Sigma-Aldrich), and separated on the basis of molecular weight under SDS-PAGE conditions (Fig. 5b), as described47, and as reported in Supplementary Methods in detail.
ELISPOT assay.
We determined the effector function of CD8+ T cells ex vivo and responses of resting CD8+ T cell responses in vitro, as described48 and as reported in Supplementary Methods in detail.
Pentamer and intracellular cytokine staining.
We stained PBMCs with allophycocyanin (APC)-labeled–HLA-A*0201 pentamers (complexed to vimentin78−87 (LLQDSVDFSL), nonmuscle myosin478−486 (QLFNHTMFI), nonmuscle myosin741−749 (VLMIKALEL) or actin266−274 (FLGMESCGI) peptide; all Proimmune Limited), APC-Cy7-labeled mAb to CD8 (BD Pharmingen), FITC-labeled perforin mAb (BD Pharmingen) and PE-labeled granzyme B mAb (PeliCluster) as described49. We obtained negative controls by staining cells with an irrelevant isotype-matched mAb. Then, we analyzed them with a FACSCanto flow cytometer and FACSDiva analysis software (Becton Dickinson). Intracellular cytokine staining (ICSS) was performed as described49, and as reported in Supplementary Methods in detail.
Cross-presentation assay.
We pulsed immature monocyte-derived DCs or myeloid DCs (3 × 104), as APCs, with increasing concentrations of caspase inhibitor–treated, caspase-treated, untreated or r-vimentin–loaded apoptotic cells, control lysed cells, soluble antigens or peptides in the presence or absence of 80 mM lactacystin (Sigma-Aldrich) in U-bottom 96-well plates for 18 h. Then we washed and cultured APCs with antigen-specific CD8+ T cells (2–3 × 104), and IFN-γ spot formation was promptly revealed after 6–8 h at 37 °C by an ELISPOT assay, as described above48. In some experiments, we pulsed iDCs with apoptotic or control cells (derived from HLA-A2− Epstein-Barr virus (EBV)-transformed B cells) that had been infected by either wild-type vaccinia virus or NS3Ag-expressing vaccinia virus (5 plaque-forming units per cell) and then cocultured them with NS31406–1415–specific CD8+ T cell clones. After 6–8 h at 37 °C, IFN-γ spot formation by CD8+ T cells was revealed by ELISPOT assay as described.
Cross-presentation experiments ex vivo.
In selective experiments, PBMCs from HIV-infected individuals or healthy donors were double-stained with pentamers and mAb to CD8 and cultured (in the presence of soluble recombinant CD40L molecules) with autologous iDCs that had been pulsed or not with apoptotic cloned T cells. After 6–8 h, we tested them for their capacity to produce IFN-γ by ICSS assay49. Cells were washed, acquired with a FACSCanto flow cytometer and analyzed with FACSDiva analysis software (Becton Dickinson).
Peptide synthesis and class I binding.
Statistical analyses.
All statistical analyses were performed with both SPSS 11.0 (SPSS Inc.) and Prism 4 (GraphPad) software using Pearson's correlation test (Fig. 3) and nonparametric Mann-Whitney U-test (Fig. 5). The differences were considered significant at P < 0.05.
Note: Supplementary information is available on the Nature Medicine website.
References
Thiede, B. & Rudel, T. Proteome analysis of apoptotic cells. Mass Spectrom. Rev. 23, 333–349 (2004).
Walker, L.S. & Abbas, A.K. The enemy within: keeping self-reactive T cells at bay in the periphery. Nat. Rev. Immunol. 2, 11–19 (2002).
Graham, K.L. & Utz, P.J. Sources of autoantigens in systemic lupus erythematosus. Curr. Opin. Rheumatol. 17, 513–517 (2005).
Mahoney, J.A. & Rosen, A. Apoptosis and autoimmunity. Curr. Opin. Immunol. 17, 583–588 (2005).
Albert, M.L. Death-defying immunity: do apoptotic cells influence antigen processing and presentation? 4, 223–231 (2004).
Bevan, M.J. Cross-priming for a secondary cytotoxic response to minor H antigens with H-2 congenic cells which do not cross-react in the cytotoxic assay. J. Exp. Med. 143, 1283–1288 (1976).
Groothuis, T.A. & Neefjes, J. The many roads to cross-presentation. J. Exp. Med. 202, 1313–1318 (2005).
Mellman, I. & Steinman, R.M. Dendritic cells: specialized and regulated antigen processing machines. Cell 106, 255–258 (2001).
Watts, C. Capture and processing of exogenous antigens for presentation on MHC molecules. Annu. Rev. Immunol. 15, 821–850 (1997).
Heath, W.R. & Carbone, F.R. Cross-presentation, dendritic cells, tolerance and immunity. Annu. Rev. Immunol. 19, 47–64 (2001).
Matzinger, P. The danger model: a renewed sense of self. Science 296, 301–305 (2002).
Steinman, R.M., Hawiger, D. & Nussenzweig, M.C. Tolerogenic dendritic cells. Annu. Rev. Immunol. 21, 685–711 (2003).
Propato, A. et al. Apoptotic cells overexpress vinculin and induce vinculin-specific cytotoxic T cell cross-priming. Nat. Med. 7, 807–813 (2001).
Grossman, Z., Meier-Schellersheim, M., Paul, W.E. & Picker, L.J. Pathogenesis of HIV infection: what the virus spares is as important as what it destroys. Nat. Med. 12, 289–295 (2006).
Douek, D.C., Picker, L.J. & Koup, R.A. T cell dynamics in HIV-1 infection. Annu. Rev. Immunol. 21, 265–304 (2003).
Silvestri, G. & Feinberg, M.B. Turnover of lymphocytes and conceptual paradigms in HIV infection. J. Clin. Invest. 112, 821–824 (2003).
Bangs, S.C., McMichael, A.J. & Xu, X.N. Bystander T cell activation - implications for HIV infection and other diseases. Trends Immunol. 27, 518–524 (2006).
Brenchley, J.M., Price, D.A. & Douek, D.C. HIV disease: fallout from a mucosal catastrophe? Nat. Immunol. 7, 235–239 (2006).
Brenchley, J.M. et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat. Med. 12, 1365–1371 (2006).
Eggena, M.P. et al. Depletion of regulatory T cells in HIV infection is associated with immune activation. J. Immunol. 174, 4407–4414 (2005).
Meffre, E. et al. Surrogate light chain expressing human peripheral B cells produce self-reactive antibodies. J. Exp. Med. 199, 145–150 (2004).
Sidney, J. et al. Majority of peptides binding HLA-A*0201 with high affinity crossreact with other A2-supertype molecules. Hum. Immunol. 62, 1200–1216 (2001).
Vanderlugt, C.L. & Miller, S.D. Epitope spreading in immune-mediated diseases: implication for immunotherapy. Nat. Rev. Immunol. 2, 85–95 (2002).
Brockstedt, E. et al. Identification of apoptosis-associated proteins in a human Burkitt lymphoma cell line. Cleavage of heterogeneous nuclear ribonucleoprotein A1 by caspase 3. J. Biol. Chem. 273, 28057–28064 (1998).
Adrain, C., Creagh, E.M., Cullen, S.P. & Martin, S.J. Caspase-dependent inactivation of proteasome function during programmed cell death in Drosophila and man. J. Biol. Chem. 279, 36923–36930 (2004).
Vicente-Manzanares, M. & Sanchez-Madrid, F. Role of the cytoskeleton during leukocyte responses. Nat. Rev. Immunol. 4, 110–122 (2004).
Accapezzato, D. et al. Chloroquine enhances human CD8+ T cell responses against soluble antigens in vivo. J. Exp. Med. 202, 817–828 (2005).
Norbury, C.C. et al. CD8+ T cell cross-priming via transfer of proteasome substrates. Science 304, 1318–1321 (2004).
Bevan, M.J. Helping the CD8+ T cell response. Nat. Rev. Immunol. 4, 595–602 (2004).
Shen, L. & Rock, K.L. Cellular protein is the source of cross-priming antigen in vivo. Proc. Natl. Acad. Sci. USA 101, 3035–3040 (2004).
Wolkers, M.C., Brouwenstijn, N., Bakker, A.H., Toebes, M. & Schumacher, T.N. Antigen bias in T cell cross-priming. Science 304, 1314–1317 (2004).
Blachere, N.E., Darnell, R.B. & Albert, M.L. Apoptotic cells deliver processed antigen to dendritic cells for cross-presentation. PLoS Biol. 3, e185 (2005).
Neijssen, J. et al. Cross-presentation by intercellular peptide transfer through gap junctions. Nature 434, 83–88 (2005).
Binder, R.J. & Srivastava, P.K. Peptides chaperoned by heat-shock proteins are a necessary and sufficient source of antigen in the cross-priming of CD8+ T cells. Nat. Immunol. 6, 593–599 (2005).
Princiotta, M.F. et al. Quantitating protein synthesis, degradation, and endogenous antigen processing. Immunity 18, 343–354 (2003).
Basta, S., Stoessel, R., Basler, M., van den Broek, M. & Groettrup, M. Cross-presentation of the long-lived lymphocytic choriomeningitis virus nucleoprotein does not require neosynthesis and is enhanced via heat shock proteins. J. Immunol. 175, 796–805 (2005).
Shi, Y., Evans, J.E. & Rock, K.L. Molecular identification of a danger signal that alerts the immune system to dying cells. Nature 425, 516–521 (2003).
Chernysheva, A.D., Kirou, K.A. & Crow, M.K. T cell proliferation induced by autologous non-T cells is a response to apoptotic cells processed by dendritic cells. J. Immunol. 169, 1241–1250 (2002).
Casares, N. et al. Caspase-dependent immunogenicity of doxorubicin-induced tumor cell death. J. Exp. Med. 202, 1691–1701 (2005).
Barnaba, V., Franco, A., Alberti, A., Benvenuto, R. & Balsano, F. Selective killing of hepatitis B envelope antigen-specific B cells by class I-restricted, exogenous antigen-specific T lymphocytes. Nature 345, 258–260 (1990).
Sallusto, F. & Lanzavecchia, A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor α. J. Exp. Med. 179, 1109–1118 (1994).
Albert, M.L. et al. Immature dendritic cells phagocytose apoptotic cells via alphavbeta5 and CD36, and cross-present antigens to cytotoxic T lymphocytes. J. Exp. Med. 188, 1359–1368 (1998).
Mann, M., Hojrup, P. & Roepstorff, P. Use of mass spectrometric molecular weight information to identify proteins in sequence databases. Biol. Mass Spectrom. 22, 338–345 (1993).
Steen, H. & Mann, M. The ABC's (and XYZ's) of peptide sequencing. Nat. Rev. Mol. Cell Biol. 5, 699–711 (2004).
Summers, D.F. & Szewczyk, B. Elution of SDS-PAGE separated proteins from immobilon membranes for use as antigens. in The Protein Protocols Handbook (ed. Walker, J.M.) 699–702 (Humana Press, Totowa, NJ, 1996).
Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72, 248–254 (1976).
Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227, 680–685 (1970).
Scognamiglio, P. et al. Presence of effector CD8+ T cells in hepatitis C virus-exposed healthy seronegative donors. J. Immunol. 162, 6681–6689 (1999).
Francavilla, V. et al. Subversion of effector CD8+ T cell differentiation in acute hepatitis C virus infection: exploring the immunological mechanisms. Eur. J. Immunol. 34, 427–437 (2004).
Acknowledgements
This work was supported by the European Union; the Fondo per gli Investimenti della Ricerca di Base and Programmi di Ricerca scientifica di rilevante Interesse Nazionale, Ministero dell'Istruzione, dell'Università e della Ricerca projects; the Ministero della Sanità-Istituto Superiore di Sanità (Progetti AIDS); the Ministero della Sanità-Ricerca finalizzata HCV; the Associazione Italiana per la Ricerca sul Cancro; the Fondazione Italiana Sclerosi Multipla; and Contributo Regione Lazio per la realizzazione di opere strutturali ed iniziative sociali, culturali e sportive di carattere locale 2006. Recombinant IL-4, CD40L-transfected J558L cells, and rabbit polyclonal antibody to αvβ5 antibody were donated by A. Lanzavecchia (Institute for Research in Biomedicine, Bellinzona, Switzerland), P. Lane (University of Birmingham Medical School, Birmingham, UK) and G. Santoni (Università di Camerino, Italy), respectively.
Author information
Authors and Affiliations
Contributions
P.M.R., C.M., M.V., T.D. and L.F. conducted proteomic and molecular analyses; L.A., D.F., A.P. and F.M. performed immunology experiments (cross-presentation, flow cytometry analyses and T cell cloning); M.P., C.M.M. and G.d'E. procured samples and recruited subjects; J.S. and A.S. synthesized peptides; V.B. conceived the study and wrote the manuscript.
Corresponding author
Supplementary information
Supplementary Text and Figures
Supplementary Figs. 1–8, Supplementary Tables 1–3, Supplementary Data 1 and 2, Supplementary Methods (PDF 2975 kb)
Rights and permissions
About this article
Cite this article
Rawson, P., Molette, C., Videtta, M. et al. Cross-presentation of caspase-cleaved apoptotic self antigens in HIV infection. Nat Med 13, 1431–1439 (2007). https://doi.org/10.1038/nm1679
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm1679
This article is cited by
-
CD8+ T cells specific for cryptic apoptosis-associated epitopes exacerbate experimental autoimmune encephalomyelitis
Cell Death & Disease (2021)
-
Incidence of autoimmune diseases in people living with HIV compared to a matched population: a cohort study
Clinical Rheumatology (2021)
-
The immune system view of the coronavirus SARS-CoV-2
Biology Direct (2020)
-
Combination of chemotherapy and PD-1 blockade induces T cell responses to tumor non-mutated neoantigens
Communications Biology (2020)
-
Dying cells actively regulate adaptive immune responses
Nature Reviews Immunology (2017)