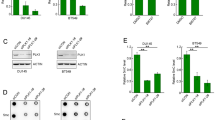
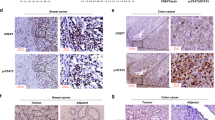
Abstract
Although STAT5A and STAT5B have some nonredundant functional properties, their distinct contributions to carcinogenesis are not clearly defined. Here we report that STAT5A expression is selectively inhibited by DNA methylation of the STAT5A gene promoter region in cells expressing the oncogenic tyrosine kinase NPM1-ALK (also known as NPM-ALK). The DNA methylation is induced by NPM1-ALK itself via STAT3, and is associated with binding to the promoter of the gene encoding MeCP2 capping protein and with lack of binding of the STAT5A gene transcription activator SP1. Reversal of methylation by the DNA methyltransferase inhibitor 5′-aza-2′-deoxycytidine restores SP1 binding and STAT5A gene expression. Notably, the induced or exogenously expressed STAT5A protein binds to the enhancer and intron 14 of the NPM1-ALK gene and triggers selective suppression of NPM1-ALK expression. These results show that NPM1-ALK induces epigenetic silencing of STAT5A gene and that STAT5A protein can act as a key tumor suppressor by reciprocally inhibiting expression of NPM1-ALK.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Accession codes
References
Moriggl, R., Sexl, V., Piekorz, R., Topham, D. & Ihle, J.N. STAT5 is required for IL-2-induced cell cycle progression of peripheral T cells. Immunity 10, 249–259 (1999).
Buitenhuis, M., Coffer, P.J. & Koenderman, L. Signal transducer and activator of transcription 5 (STAT5). Int. J. Biochem. Cell Biol. 36, 2120–2124 (2004).
Migone, T.S. et al. Constitutively activated Jak-STAT pathway in T cells transformed with HTLV-I. Science 269, 79–81 (1995).
Xu, X. et al. Constitutive activation of different Jak tyrosine kinases in human T cell leukemia virus type 1 (HTLV-1) Tax protein or virus-transformed cells. J. Clin. Invest. 96, 1548–1555 (1995).
Zhang, Q. et al. Activation of Jak/STAT proteins involved in signal transduction pathway mediated by receptor for interleukin 2 in malignant T lymphocytes derived from cutaneous anaplastic large T-cell lymphoma and Sezary syndrome. Proc. Natl. Acad. Sci. USA 93, 9148–9153 (1996).
Nieborowska-Skorska, M. et al. Role of signal transducer and activator of transcription 5 in nucleophosmin/ anaplastic lymphoma kinase-mediated malignant transformation of lymphoid cells. Cancer Res. 61, 6517–6523 (2001).
Gouilleux-Gruart, V. et al. STAT-related transcription factors are constitutively activated in peripheral blood cells from acute leukemia patients. Blood 87, 1692–1697 (1996).
Nieborowska-Skorska, M. et al. Signal transducer and Activator of Transcription (STAT)5 activation by BCR/ABL is dependent on intact src homology (SH3) and SH2 domains of BCR/ABL and is required for leukemogenesis. J. Exp. Med. 189, 1229–1242 (1999).
Clevenger, C.V. Roles and Regulation of STAT Family Transcription Factors in Human Breast Cancer. Am. J. Pathol. 165, 1449–1460 (2004).
Lai, S.Y. et al. Erythropoietin-mediated activation of JAK-STAT signaling contributes to cellular invasion in head and neck squamous cell carcinoma. Oncogene 24, 4442–4449 (2005).
Liu, X. et al. STAT5A is mandatory for adult mammary gland development and lactogenesis. Genes Dev. 11, 179–186 (1997).
Nakajima, H. et al. An indirect effect of STAT5A in IL-2-induced proliferation: a critical role for STAT5A in IL-2-mediated IL-2 receptor alpha chain induction. Immunity 7, 691–701 (1997).
Davey, H.W., Park, S.H., Grattan, D.R., McLachlan, M.J. & Waxman, D.J. STAT5B-deficient mice are growth hormone pulse-resistant. Role of STAT5B in sex-specific liver p450 expression. J. Biol. Chem. 274, 35331–35336 (1999).
Imada, K. et al. STAT5B is essential for natural killer cell-mediated proliferation and cytolytic activity. J. Exp. Med. 188, 2067–2074 (1998).
Cui, Y. et al. Inactivation of STAT5 in mouse mammary epithelium during pregnancy reveals distinct functions in cell proliferation, survival, and differentiation. Mol. Cell. Biol. 24, 8037–8047 (2004).
Herman, J.G. & Baylin, S.B. Gene silencing in cancer in association with promoter hypermethylation. N. Engl. J. Med. 349, 2042–2054 (2003).
Wade, P.A. Methyl CpG-binding proteins: coupling chromatin architecture to gene regulation. Oncogene 20, 3166–3173 (2001).
Wasik, M.A. Expression of anaplastic lymphoma kinase (ALK) in non-Hodgkin's lymphomas and other malignancies: biological, diagnostic and clinical implications. Am. J. Clin. Pathol. 118, S81–S92 (2002).
Morris, S.W. et al. Fusion of a kinase gene, ALK, to a nucleolar protein gene, NPM, in non-Hodgkin's lymphoma. Science 263, 1281–1284 (1994).
Shiota, M. et al. Hyperphosphorylation of a novel 80 kDa protein-tyrosine kinase similar to Ltk in a human Ki-1 lymphoma cell line, AMS3. Oncogene 9, 1567–1574 (1994).
Fujimoto, J. et al. Characterization of the transforming activity of p80, a hyperphosphorylated protein in a Ki-1 lymphoma cell line with chromosomal translocation t(2;5). Proc. Natl. Acad. Sci. USA 93, 4181–4186 (1996).
Bischof, D., Pulford, K., Mason, D.Y. & Morris, S.W. Role of the nucleophosmin (NPM) portion of the non-Hodgkins lymphoma-associated NPM-anaplastic lymphoma kinase fusion protein in oncogenesis. Mol. Cell. Biol. 17, 2312–2325 (1997).
Kuefer, M.U. et al. Retrovirus-mediated gene transfer of NPM-ALK causes lymphoid malignancy in mice. Blood 90, 2901–2910 (1997).
Chiarle, R. et al. NPM-ALK transgenic mice spontaneously develop T-cell lymphomas and plasma cell tumors. Blood 101, 1919–1927 (2003).
Zhang, Q. et al. Multilevel dysregulation of STAT3 activation in anaplastic lymphoma kinase-positive T/null-cell lymphoma. J. Immunol. 168, 466–474 (2002).
Hendry, L. & John, S. Regulation of STAT signaling by proteolytic processing. Eur. J. Biochem. 271, 4613–4620 (2004).
Ambrosio, R. et al. The structure of human STAT5A and B genes reveals two regions of nearly identical sequence and an alternative tissue specific STAT5B promoter. Gene 285, 311–318 (2002).
Crispi, S. et al. Characterization of the human STAT5A and STAT5B promoters: evidence of a positive and negative mechanism of transcriptional regulation. FEBS Lett. 562, 27–34 (2004).
Soldaini, E. et al. DNA Binding Site Selection of Dimeric and Tetrameric STAT5 Proteins Reveals a Large Repertoire of Divergent Tetrameric STAT5A Binding Sites. Mol. Cell. Biol. 20, 389–401 (2000).
Ehret, G.B. et al. DNA Binding Specificity of Different STAT Proteins. J. Biol. Chem. 276, 6675–6688 (2001).
Carter, D., Chakalova, L., Osborne, C.S., Dai, Y.F. & Fraser, P. Long-range chromatin regulatory interactions in vivo. Nat. Genet. 32, 623–626 (2002).
Bondarenko, V.A., Liu, Y.V., Jiang, Y.I. & Studitsky, V.M. Communication over a large distance: enhancers and insulators. Biochem. Cell Biol. 81, 241–251 (2003).
Moriggl, R. et al. STAT5 tetramer formation is associated with leukemogenesis. Cancer Cell 7, 87–99 (2005).
Zhang, Q. et al. STAT3 induces transcription of the DNA methyltransferase 1 gene (DNMT1) in malignant T lymphocytes. Blood 108, 1058–1064 (2006).
Zhang, Q. et al. STAT3- and DNA methyltransferase 1-mediated epigenetic silencing of SHP-1 tyrosine phosphatase tumor suppressor gene in malignant T lymphocytes. Proc. Natl. Acad. Sci. USA 102, 6948–6953 (2005).
Yu, H. & Jove, R. The STATs of cancer–new molecular targets come of age. Nat. Rev. Cancer 4, 97–105 (2004).
Nevalainen, M.T. et al. Signal transducer and activator of transcription-5 activation and breast cancer prognosis. J. Clin. Oncol. 22, 2053–2060 (2004).
Sultan, A.S. et al. STAT5 promotes homotypic adhesion and inhibits invasive characteristics of human breast cancer cells. Oncogene 24, 746–760 (2005).
Marzec, M. et al. Inhibition of anaplastic lymphoma kinase (ALK) enzymatic activity in T-cell lymphoma cells induces apoptosis and suppresses proliferation and STAT3 phosphorylation independently of Jak3. Lab. Invest. 85, 1544–1554 (2005).
Wan, W. et al. Anaplastic lymphoma kinase activity is essential for the proliferation and survival of anaplastic large-cell lymphoma cells. Blood 107, 1617–1623 (2006).
Galkin, A.V. et al. Identification of NVP-TAE684, a potent, selective and efficacious inhibitor of NPM/ALK. Proc. Natl. Acad. Sci. USA 104, 270–275 (2007).
Hehlmann, R., Berger, U. & Hochhaus, A. Chronic myeloid leukemia: a model for oncology. Ann. Hematol. 84, 487–497 (2005).
Claus, R. & Lübbert, M. Epigenetic targets in hematopoietic malignancies. Oncogene 22, 6489–6496 (2003).
Wlodarski, P. et al. Activation of mTOR in transformed B lymphocytes is nutrient-dependent but independent of Akt, MEK, IGF-I, and serum. Cancer Res. 65, 7800–7808 (2005).
Acknowledgements
This work was supported in part by US National Cancer Institute grants R01-CA89194 and R01-CA96856.
Author information
Authors and Affiliations
Corresponding authors
Supplementary information
Supplementary Text and Figures
Supplementary Information Title Page and Supplementary Figs. 1–3 (PDF 1717 kb)
Rights and permissions
About this article
Cite this article
Zhang, Q., Wang, H., Liu, X. et al. STAT5A is epigenetically silenced by the tyrosine kinase NPM1-ALK and acts as a tumor suppressor by reciprocally inhibiting NPM1-ALK expression. Nat Med 13, 1341–1348 (2007). https://doi.org/10.1038/nm1659
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm1659
This article is cited by
-
Chimeric kinase ALK induces expression of NAMPT and selectively depends on this metabolic enzyme to sustain its own oncogenic function
Leukemia (2023)
-
PDGFRβ promotes oncogenic progression via STAT3/STAT5 hyperactivation in anaplastic large cell lymphoma
Molecular Cancer (2022)
-
Epigenetic alterations and advancement of treatment in peripheral T-cell lymphoma
Clinical Epigenetics (2020)
-
Unphosphorylated STAT3 in heterochromatin formation and tumor suppression in lung cancer
BMC Cancer (2020)
-
Epigenetic downregulation of STAT6 increases HIF-1α expression via mTOR/S6K/S6, leading to enhanced hypoxic viability of glioma cells
Acta Neuropathologica Communications (2019)