Abstract
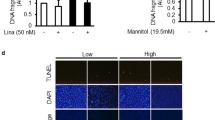
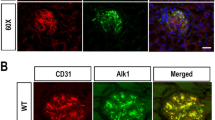
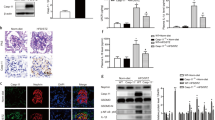
Data providing direct evidence for a causative link between endothelial dysfunction, microvascular disease and diabetic end-organ damage are scarce. Here we show that activated protein C (APC) formation, which is regulated by endothelial thrombomodulin, is reduced in diabetic mice and causally linked to nephropathy. Thrombomodulin-dependent APC formation mediates cytoprotection in diabetic nephropathy by inhibiting glomerular apoptosis. APC prevents glucose-induced apoptosis in endothelial cells and podocytes, the cellular components of the glomerular filtration barrier. APC modulates the mitochondrial apoptosis pathway via the protease-activated receptor PAR-1 and the endothelial protein C receptor EPCR in glucose-stressed cells. These experiments establish a new pathway, in which hyperglycemia impairs endothelial thrombomodulin-dependent APC formation. Loss of thrombomodulin-dependent APC formation interrupts cross-talk between the vascular compartment and podocytes, causing glomerular apoptosis and diabetic nephropathy. Conversely, maintaining high APC levels during long-term diabetes protects against diabetic nephropathy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Renal Data System: USRDS 1998 Annual Data Report (NIH Publ. No. 98–3176) (US Government Printing Office, Washington, DC, 1998).
Wendt, T.M. et al. RAGE drives the development of glomerulosclerosis and implicates podocyte activation in the pathogenesis of diabetic nephropathy. Am. J. Pathol. 162, 1123–1137 (2003).
Haraldsson, B. & Sorensson, J. Why do we not all have proteinuria? An update of our current understanding of the glomerular barrier. News Physiol. Sci. 19, 7–10 (2004).
Dalla Vestra, M. et al. Is podocyte injury relevant in diabetic nephropathy? Studies in patients with type 2 diabetes. Diabetes 52, 1031–1035 (2003).
Meyer, T.W., Bennett, P.H. & Nelson, R.G. Podocyte number predicts long-term urinary albumin excretion in Pima Indians with Type II diabetes and microalbuminuria. Diabetologia 42, 1341–1344 (1999).
Esmon, C.T. Inflammation and the activated protein C anticoagulant pathway. Semin. Thromb. Hemost. 32 (Suppl. 1), 49–60 (2006).
Riewald, M., Petrovan, R.J., Donner, A., Mueller, B.M. & Ruf, W. Activation of endothelial cell protease activated receptor 1 by the protein C pathway. Science 296, 1880–1882 (2002).
Cheng, T. et al. Activated protein C blocks p53-mediated apoptosis in ischemic human brain endothelium and is neuroprotective. Nat. Med. 9, 338–342 (2003).
Feistritzer, C. & Riewald, M. Endothelial barrier protection by activated protein C through PAR1-dependent sphingosine 1-phosphate receptor-1 crossactivation. Blood 105, 3178–3184 (2005).
Finigan, J.H. et al. Activated protein C mediates novel lung endothelial barrier enhancement: role of sphingosine 1-phosphate receptor transactivation. J. Biol. Chem. 280, 17286–17293 (2005).
Borcea, V. et al. Influence of ramipril on the course of plasma thrombomodulin in patients with diabetes mellitus. Vasa 28, 172–180 (1999).
Fujiwara, Y., Tagami, S. & Kawakami, Y. Circulating thrombomodulin and hematological alterations in type 2 diabetic patients with retinopathy. J. Atheroscler. Thromb. 5, 21–28 (1998).
Weiler-Guettler, H. et al. A targeted point mutation in thrombomodulin generates viable mice with a prethrombotic state. J. Clin. Invest. 101, 1983–1991 (1998).
Hall, S.W., Nagashima, M., Zhao, L., Morser, J. & Leung, L.L. Thrombin interacts with thrombomodulin, protein C, and thrombin-activatable fibrinolysis inhibitor via specific and distinct domains. J. Biol. Chem. 274, 25510–25516 (1999).
Festa, A. et al. Inflammation and microalbuminuria in nondiabetic and type 2 diabetic subjects: The Insulin Resistance Atherosclerosis Study. Kidney Int. 58, 1703–1710 (2000).
Isermann, B. et al. The thrombomodulin–protein C system is essential for the maintenance of pregnancy. Nat. Med. 9, 331–337 (2003).
Mosnier, L.O. & Griffin, J.H. Inhibition of staurosporine-induced apoptosis of endothelial cells by activated protein C requires protease-activated receptor-1 and endothelial cell protein C receptor. Biochem. J. 373, 65–70 (2003).
Tewari, M. et al. Yama/CPP32 beta, a mammalian homolog of CED-3, is a CrmA-inhibitable protease that cleaves the death substrate poly(ADP-ribose) polymerase. Cell 81, 801–809 (1995).
Wang, J. et al. Minocycline up-regulates Bcl-2 and protects against cell death in mitochondria. J. Biol. Chem. 279, 19948–19954 (2004).
Kelly, K.J. et al. Minocycline inhibits apoptosis and inflammation in a rat model of ischemic renal injury. Am. J. Physiol. Renal Physiol. 287, F760–F766 (2004).
Brownlee, M. Biochemistry and molecular cell biology of diabetic complications. Nature 414, 813–820 (2001).
Zou, M.H., Shi, C. & Cohen, R.A. High glucose via peroxynitrite causes tyrosine nitration and inactivation of prostacyclin synthase that is associated with thromboxane/prostaglandin H(2) receptor-mediated apoptosis and adhesion molecule expression in cultured human aortic endothelial cells. Diabetes 51, 198–203 (2002).
Yamaji, K. et al. Activated protein C, a natural anticoagulant protein, has antioxidant properties and inhibits lipid peroxidation and advanced glycation end products formation. Thromb. Res. 115, 319–325 (2005).
Riewald, M. & Ruf, W. Protease-activated receptor-1 signaling by activated protein C in cytokine-perturbed endothelial cells is distinct from thrombin signaling. J. Biol. Chem. 280, 19808–19814 (2005).
Goligorsky, M.S., Chen, J. & Brodsky, S. Workshop: endothelial cell dysfunction leading to diabetic nephropathy: focus on nitric oxide. Hypertension 37, 744–748 (2001).
Endemann, D.H. & Schiffrin, E.L. Endothelial dysfunction. J. Am. Soc. Nephrol. 15, 1983–1992 (2004).
Murata, I. et al. Apoptotic cell loss following cell proliferation in renal glomeruli of Otsuka Long-Evans Tokushima Fatty rats, a model of human type 2 diabetes. Am. J. Nephrol. 22, 587–595 (2002).
Kumar, D., Robertson, S. & Burns, K.D. Evidence of apoptosis in human diabetic kidney. Mol. Cell. Biochem. 259, 67–70 (2004).
Baba, K. et al. Involvement of apoptosis in patients with diabetic nephropathy: A study on plasma soluble Fas levels and pathological findings. Nephrology (Carlton) 9, 94–99 (2004).
Liaw, P.C. et al. Patients with severe sepsis vary markedly in their ability to generate activated protein C. Blood 104, 3958–3964 (2004).
Macias, W.L. et al. New insights into the protein C pathway: potential implications for the biological activities of drotrecogin alfa (activated). Crit. Care 9 Suppl 4, S38–S45 (2005).
Glaser, C.B. et al. Oxidation of a specific methionine in thrombomodulin by activated neutrophil products blocks cofactor activity. A potential rapid mechanism for modulation of coagulation. J. Clin. Invest. 90, 2565–2573 (1992).
Boehme, M.W. et al. Release of thrombomodulin from endothelial cells by concerted action of TNF-alpha and neutrophils: in vivo and in vitro studies. Immunology 87, 134–140 (1996).
Mosnier, L.O., Gale, A.J., Yegneswaran, S. & Griffin, J.H. Activated protein C variants with normal cytoprotective but reduced anticoagulant activity. Blood 104, 1740–1744 (2004).
Bernard, G.R. et al. Safety assessment of drotrecogin alfa (activated) in the treatment of adult patients with severe sepsis. Crit. Care 7, 155–163 (2003).
Taylor, F.B. & Kinasewitz, G. Activated protein C in sepsis. J. Thromb. Haemost. 2, 708–717 (2004).
Chae, S.S., Proia, R.L. & Hla, T. Constitutive expression of the S1P1 receptor in adult tissues. Prostaglandins Other Lipid Mediat. 73, 141–150 (2004).
Virag, L. & Szabo, C. The therapeutic potential of poly(ADP-ribose) polymerase inhibitors. Pharmacol. Rev. 54, 375–429 (2002).
Cipriani, G. et al. Nuclear poly(ADP-ribose) polymerase-1 rapidly triggers mitochondrial dysfunction. J. Biol. Chem. 280, 17227–17234 (2005).
Alano, C.C., Kauppinen, T.M., Valls, A.V. & Swanson, R.A. Minocycline inhibits poly(ADP-ribose) polymerase-1 at nanomolar concentrations. Proc. Natl. Acad. Sci. USA 103, 9685–9690 (2006).
Szabo, C., Biser, A., Benko, R., Bottinger, E. & Susztak, K. Poly(ADP-ribose) polymerase inhibitors ameliorate nephropathy of type 2 diabetic Leprdb/db mice. Diabetes 55, 3004–3012 (2006).
Kiss, L. et al. The pathogenesis of diabetic complications: the role of DNA injury and poly(ADP-ribose) polymerase activation in peroxynitrite-mediated cytotoxicity. Mem. Inst. Oswaldo Cruz 100 (Suppl. 1), 29–37 (2005).
Richardson, M.A., Gerlitz, B. & Grinnell, B.W. Enhancing protein C interaction with thrombin results in a clot-activated anticoagulant. Nature 360, 261–264 (1992).
Chen, M. et al. Minocycline inhibits caspase-1 and caspase-3 expression and delays mortality in a transgenic mouse model of Huntington disease. Nat. Med. 6, 797–801 (2000).
Isermann, B. et al. Endothelium-specific loss of murine thrombomodulin disrupts the protein C anticoagulant pathway and causes juvenile-onset thrombosis. J. Clin. Invest. 108, 537–546 (2001).
Kerlin, B.A. et al. Survival advantage associated with heterozygous Factor V Leiden mutation in patients with severe sepsis and in mouse endotoxemia. Blood 102, 3085–3092 (2003).
Calkin, A.C. et al. PPAR-α and -γ agonists attenuate diabetic kidney disease in the apolipoprotein E knockout mouse. Nephrol. Dial. Transplant. 21, 2399–2405 (2006).
Mundel, P. et al. Rearrangements of the cytoskeleton and cell contacts induce process formation during differentiation of conditionally immortalized mouse podocyte cell lines. Exp. Cell Res. 236, 248–258 (1997).
Asanuma, K. et al. Synaptopodin regulates the actin-bundling activity of alpha-actinin in an isoform-specific manner. J. Clin. Invest. 115, 1188–1198 (2005).
Acknowledgements
We thank S. Huntscha and C. Luckner for technical support. This work was supported by grants from the Deutsche Forschungsgemeinschaft (IS 67/2-1; IS 67/2-2) to B.I., a grant from the Novartis Stiftung to B.I. and T.C., a grant from the Lautenschläger Stiftung, and a European Association for the Study of Diabetes grant to A.B.
Author information
Authors and Affiliations
Contributions
B.I. conceptually designed, conducted and interpreted the experimental work and wrote the manuscript; I.A.V. did in vivo and ex vivo work; T.M. did ex vivo and in vitro work, S.H. did in vitro work; M.K. and J.B. did ex vivo work; M.A.F.C. did transgenic mouse work; M.Z. contributed to data analyses; E.B. and T.C. performed image analyses; J.O. contributed to podocyte in vitro work, B.G., D.T.B. and B.W.G. performed in vitro analyses of the D167F/D172K PC mutant; C.T.E. provided antibodies and helped interpret experimental work; H.W. helped generate APChigh mice and made ThbdPro/Pro mice available; A.B. helped design experiments; P.P.N. designed experiments and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
C.T.E. holds a patent and license for the use of APC in sepsis and production of protein C from plasma. He also has patents on detecting circulating APC levels. B.W.G., D.T.B. and B.G. are employees of Lilly Research Laboratories, a division of Eli Lilly & Co., which produces recombinant human protein C for the treatment of severe sepsis.
Supplementary information
Supplementary Text and Figures
Supplementary Tables 1–5, Supplementary Methods, Supplementary Figs. 1–4 (PDF 377 kb)
Rights and permissions
About this article
Cite this article
Isermann, B., Vinnikov, I., Madhusudhan, T. et al. Activated protein C protects against diabetic nephropathy by inhibiting endothelial and podocyte apoptosis. Nat Med 13, 1349–1358 (2007). https://doi.org/10.1038/nm1667
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm1667
This article is cited by
-
Screening of Diabetic Nephropathy Progression-Related Genes Based on Weighted Gene Co-expression Network Analysis
Biochemical Genetics (2023)
-
Antagonizing the irreversible thrombomodulin-initiated proteolytic signaling alleviates age-related liver fibrosis via senescent cell killing
Cell Research (2023)
-
Aptamer loaded superparamagnetic beads for selective capturing and gentle release of activated protein C
Scientific Reports (2022)
-
Cellular crosstalk of glomerular endothelial cells and podocytes in diabetic kidney disease
Journal of Cell Communication and Signaling (2022)
-
The role of microRNA-155 in glomerular endothelial cell injury induced by high glucose
Molecular Biology Reports (2022)