Abstract
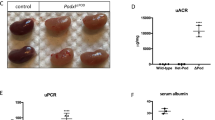
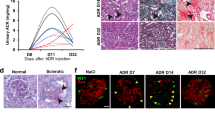
Rapidly progressive glomerulonephritis (RPGN) is a clinical syndrome characterized by loss of renal function within days to weeks and by glomerular crescents on biopsy. The pathogenesis of this disease is unclear, but circulating factors are believed to have a major role1,2. Here, we show that deletion of the Von Hippel–Lindau gene (Vhlh) from intrinsic glomerular cells of mice is sufficient to initiate a necrotizing crescentic glomerulonephritis and the clinical features that accompany RPGN. Loss of Vhlh leads to stabilization of hypoxia-inducible factor α subunits (HIFs). Using gene expression profiling, we identified de novo expression of the HIF target gene Cxcr4 (ref. 3) in glomeruli from both mice and humans with RPGN. The course of RPGN is markedly improved in mice treated with a blocking antibody to Cxcr4, whereas overexpression of Cxcr4 alone in podocytes of transgenic mice is sufficient to cause glomerular disease. Collectively, these results indicate an alternative mechanism for the pathogenesis of RPGN and glomerular disease in an animal model and suggest novel molecular pathways for intervention in this disease.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Falk, R.J., Terrell, R.S., Charles, L.A. & Jennette, J.C. Anti-neutrophil cytoplasmic autoantibodies induce neutrophils to degranulate and produce oxygen radicals in vitro. Proc. Natl. Acad. Sci. USA 87, 4115–4119 (1990).
Xiao, H. et al. Antineutrophil cytoplasmic autoantibodies specific for myeloperoxidase cause glomerulonephritis and vasculitis in mice. J. Clin. Invest. 110, 955–963 (2002).
Staller, P. et al. Chemokine receptor CXCR4 downregulated by von Hippel-Lindau tumour suppressor pVHL. Nature 425, 307–311 (2003).
Somlo, S. & Mundel, P. Getting a foothold in nephrotic syndrome. Nat. Genet. 24, 333–335 (2000).
Eremina, V. et al. Glomerular-specific alterations of VEGF-A expression lead to distinct congenital and acquired renal diseases. J. Clin. Invest. 111, 707–716 (2003).
Davies, D.J., Moran, J.E., Niall, J.F. & Ryan, G.B. Segmental necrotising glomerulonephritis with antineutrophil antibody: possible arbovirus aetiology? Br. Med. J. (Clin. Res. Ed.) 285, 606 (1982).
Hedger, N., Stevens, J., Drey, N., Walker, S. & Roderick, P. Incidence and outcome of pauci-immune rapidly progressive glomerulonephritis in Wessex, UK: a 10-year retrospective study. Nephrol. Dial. Transplant. 15, 1593–1599 (2000).
Galban, S. et al. von Hippel-Lindau protein-mediated repression of tumor necrosis factor alpha translation revealed through use of cDNA arrays. Mol. Cell. Biol. 23, 2316–2328 (2003).
Timoshanko, J.R., Sedgwick, J.D., Holdsworth, S.R. & Tipping, P.G. Intrinsic renal cells are the major source of tumor necrosis factor contributing to renal injury in murine crescentic glomerulonephritis. J. Am. Soc. Nephrol. 14, 1785–1793 (2003).
Li, C.G. et al. Serum levels of vascular endothelial growth factor (VEGF) are markedly elevated in patients with Wegener's granulomatosis. Br. J. Rheumatol. 37, 1303–1306 (1998).
Sommer, M. et al. Rapidly progressive glomerulonephritis in a patient with advanced renal cell carcinoma. Nephrol. Dial. Transplant. 13, 2107–2109 (1998).
Kagan, A., Sinay-Trieman, L., Czernobilsky, B., Barzilai, N. & Bar-Khayim, Y. Is the association between crescentic glomerulonephritis and renal cell carcinoma coincidental? Nephron 65, 642–643 (1993).
Haase, V.H., Glickman, J.N., Socolovsky, M. & Jaenisch, R. Vascular tumors in livers with targeted inactivation of the von Hippel-Lindau tumor suppressor. Proc. Natl. Acad. Sci. USA 98, 1583–1588 (2001).
Moeller, M.J. et al. Podocytes populate cellular crescents in a murine model of inflammatory glomerulonephritis. J. Am. Soc. Nephrol. 15, 61–67 (2004).
Novak, A., Guo, C., Yang, W., Nagy, A. & Lobe, C.G.Z. EG, a double reporter mouse line that expresses enhanced green fluorescent protein upon Cre-mediated excision. Genesis 28, 147–155 (2000).
Zagzag, D. et al. Stromal cell-derived factor-1alpha and CXCR4 expression in hemangioblastoma and clear cell-renal cell carcinoma: von Hippel-Lindau loss-of-function induces expression of a ligand and its receptor. Cancer Res. 65, 6178–6188 (2005).
Maynard, M.A. & Ohh, M. Von Hippel-Lindau tumor suppressor protein and hypoxia-inducible factor in kidney cancer. Am. J. Nephrol. 24, 1–13 (2004).
Kucia, M. et al. Trafficking of normal stem cells and metastasis of cancer stem cells involve similar mechanisms: pivotal role of the SDF-1-CXCR4 axis. Stem Cells 23, 879–894 (2005).
Grone, H.J. et al. Spatial and temporally restricted expression of chemokines and chemokine receptors in the developing human kidney. J. Am. Soc. Nephrol. 13, 957–967 (2002).
Petit, I. et al. G-CSF induces stem cell mobilization by decreasing bone marrow SDF-1 and up-regulating CXCR4. Nat. Immunol. 3, 687–694 (2002).
Zhang, W.B. et al. A point mutation that confers constitutive activity to CXCR4 reveals that T140 is an inverse agonist and that AMD3100 and ALX40–4C are weak partial agonists. J. Biol. Chem. 277, 24515–24521 (2002).
Belteki, G. et al. Conditional and inducible transgene expression in mice through the combinatorial use of Cre-mediated recombination and tetracycline induction. Nucleic Acids Res. 33, e51 (2005).
Eremina, V., Wong, M.A., Cui, S., Schwartz, L. & Quaggin, S.E. Glomerular-specific gene excision in vivo. J. Am. Soc. Nephrol. 13, 788–793 (2002).
Cui, S., Li, C., Ema, M., Weinstein, J. & Quaggin, S.E. Rapid isolation of glomeruli coupled with gene expression profiling identifies downstream targets in Pod1 knockout mice. J. Am. Soc. Nephrol. 16, 3247–3255 (2005).
Wada, T., Pippin, J.W., Terada, Y. & Shankland, S.J. The cyclin-dependent kinase inhibitor p21 is required for TGF-beta1-induced podocyte apoptosis. Kidney Int. 68, 1618–1629 (2005).
Cohen, C.D., Frach, K., Schlondorff, D. & Kretzler, M. Quantitative gene expression analysis in renal biopsies: a novel protocol for a high-throughput multicenter application. Kidney Int. 61, 133–140 (2002).
Acknowledgements
The authors thank D. Vukasovic for secretarial assistance and the Centre for Modelling Human Diseases for biochemical assays in the mice. We also thank B. Pressler (University of North Carolina at Chapel Hill) for performing ANCA assays, Y. Wang (Samuel Lunenfeld Research Institute) for help in LCM isolation, S. Peiper (Institute of Molecular Medicine and Genetics, Georgia) for providing the constitutively active Cxcr4 constructs, V. Eremina for technical assistance, K. Kamel (St. Michael's Hospital, Toronto) and D. Cattran (Toronto Hospital) for critically reviewing the manuscript. S.E.Q. is the recipient of a Canada Research Chair Tier II, and a Premier's Research of Excellence Award. This work was funded by Canadian Institute of Health Research grant MOP 77756, National Cancer Institute of Canada grant #16002 and Emerald Foundation grant (to S.E.Q.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
The renal glomerulus. (PDF 68 kb)
Supplementary Fig. 2
Generation of transgenic mouse lines. (PDF 340 kb)
Supplementary Fig. 3
PCNA staining. (PDF 94 kb)
Supplementary Fig. 4
Hif1a protein is increased in podocytes from VhlhloxP/loxP Pod-Cre mice. (PDF 165 kb)
Supplementary Fig. 5
Glomerular expression of Hif target genes. (PDF 259 kb)
Supplementary Fig. 6
Expression of the Hif target gene Sdf1 in glomeruli of wild-type and mutant mice. (PDF 119 kb)
Supplementary Fig. 7
Kaplan-Meier survival curves for mutant Vhlh mice treated with blocking antibody to Cxcr4 or vehicle. (PDF 48 kb)
Supplementary Fig. 8
mRNA expression analysis of VHL-HIF pathway molecular targets in glomeruli from patients with pauci-immune RPGN, IgA nephritis or no glomerular disease. (PDF 159 kb)
Supplementary Table 1
Gene expression patterns in human RPGN compared with expression in glomeruli from VhlhloxP/loxP Pod-Cre mice. (PDF 96 kb)
Rights and permissions
About this article
Cite this article
Ding, M., Cui, S., Li, C. et al. Loss of the tumor suppressor Vhlh leads to upregulation of Cxcr4 and rapidly progressive glomerulonephritis in mice. Nat Med 12, 1081–1087 (2006). https://doi.org/10.1038/nm1460
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm1460
This article is cited by
-
PGK1 contributes to tumorigenesis and sorafenib resistance of renal clear cell carcinoma via activating CXCR4/ERK signaling pathway and accelerating glycolysis
Cell Death & Disease (2022)
-
Detrimental effects of hypoxia on glomerular podocytes
Journal of Physiology and Biochemistry (2021)
-
Recent advances in understanding the role of hypoxia-inducible factor 1α in renal fibrosis
International Urology and Nephrology (2020)
-
Mechanisms of hypoxia signalling: new implications for nephrology
Nature Reviews Nephrology (2019)
-
Coro2b, a podocyte protein downregulated in human diabetic nephropathy, is involved in the development of protamine sulphate-induced foot process effacement
Scientific Reports (2019)