Abstract
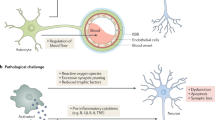
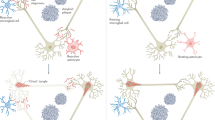
Alzheimer disease is a progressive dementia with unknown etiology that affects a growing number of the aging population. Increased expression of inflammatory mediators in postmortem brains of people with Alzheimer disease has been reported, and epidemiological studies link the use of anti-inflammatory drugs with reduced risk for the disorder. On the initial basis of this kind of evidence, inflammation has been proposed as a possible cause or driving force of Alzheimer disease. If true, this could have important implications for the development of new treatments. Alternatively, inflammation could simply be a byproduct of the disease process and may not substantially alter its course. Or components of the inflammatory response might even be beneficial and slow the disease. To address these possibilities, we need to determine whether inflammation in Alzheimer disease is an early event, whether it is genetically linked with the disease and whether manipulation of inflammatory pathways changes the course of the pathology. Although there is still little evidence that inflammation triggers or promotes Alzheimer disease, increasing evidence from mouse models suggests that certain inflammatory mediators are potent drivers of the disease. Related factors, on the other hand, elicit beneficial responses and can reduce disease.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Glenner, G.G. & Wong, C.W. Alzheimer's disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem. Biophys. Res. Commun. 120, 885–890 (1984).
Grundke-Iqbal, I. et al. Abnormal phosphorylation of the microtubule-associated protein τ (tau) in Alzheimer cytoskeletal pathology. Proc. Natl. Acad. Sci. USA 83, 4913–4917 (1986).
Goate, A. et al. Segregation of a missense mutation in the amyloid precursor protein gene with familial Alzheimer's disease. Nature 349, 704–706 (1991).
Sherrington, R. et al. Cloning of a gene bearing missense mutations in early-onset familial Alzheimer's disease. Nature 375, 754–760 (1995).
Akiyama, H. et al. Inflammation and Alzheimer's disease. Neurobiol. Aging 21, 383–421 (2000).
Wyss-Coray, T. & Mucke, L. Inflammation in neurodegenerative disease: a double-edged sword. Neuron 35, 419–432 (2002).
Iqbal, K. et al. Subgroups of Alzheimer's disease based on cerebrospinal fluid molecular markers. Ann. Neurol. 58, 748–757 (2005).
Eikelenboom, P. & Stam, F.C. Immunoglobulins and complement factors in senile plaques. An immunoperoxidase study. Acta Neuropathol. (Berl.) 57, 239–242 (1982).
McGeer, P.L., Itagaki, S., Boyes, B.E. & McGeer, E.G. Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson's and Alzheimer's disease brains. Neurology 38, 1285–1291 (1988).
Katsel, P.L., Davis, K.L. & Haroutunian, V. Large-scale microarray studies of gene expression in multiple regions of the brain in schizophrenia and Alzheimer's disease. Int. Rev. Neurobiol. 63, 41–82 (2005).
Blalock, E.M. et al. Harnessing the power of gene microarrays for the study of brain aging and Alzheimer's disease: statistical reliability and functional correlation. Ageing Res. Rev. 4, 481–512 (2005).
Colangelo, V. et al. Gene expression profiling of 12633 genes in Alzheimer hippocampal CA1: transcription and neurotrophic factor down-regulation and up-regulation of apoptotic and pro-inflammatory signaling. J. Neurosci. Res. 70, 462–473 (2002).
Groom, G.N., Junck, L., Foster, N.L., Frey, K.A. & Kuhl, D.E. PET of peripheral benzodiazepine binding sites in the microgliosis of Alzheimer's disease. J. Nucl. Med. 36, 2207–2210 (1995).
Cagnin, A. et al. In-vivo measurement of activated microglia in dementia. Lancet 358, 461–467 (2001).
Versijpt, J.J. et al. Assessment of neuroinflammation and microglial activation in Alzheimer's disease with radiolabelled PK11195 and single photon emission computed tomography. A pilot study. Eur. Neurol. 50, 39–47 (2003).
Mahley, R.W. & Rall, S.C., Jr. Apolipoprotein E: far more than a lipid transport protein. Annu. Rev. Genomics Hum. Genet. 1, 507–537 (2000).
Mahley, R. & Huang, Y. Apolipoprotein E: from atherosclerosis to Alzheimer's disease and beyond. Curr. Opin. Lipidol. 10, 207–217 (1999).
Lynch, J.R. et al. APOE genotype and an ApoE-mimetic peptide modify the systemic and central nervous system inflammatory response. J. Biol. Chem. 278, 48529–48533 (2003).
van den Elzen, P. et al. Apolipoprotein-mediated pathways of lipid antigen presentation. Nature 437, 906–910 (2005).
Kanter, J.L. et al. Lipid microarrays identify key mediators of autoimmune brain inflammation. Nat. Med. 12, 138–143 (2006).
Chapman, J. et al. APOE genotype is a major predictor of long-term progression of disability in MS. Neurology 56, 312–316 (2001).
McGeer, P.L., McGeer, E., Rogers, J. & Sibley, J. Anti-inflammatory drugs and Alzheimer disease. Lancet 335, 1037 (1990).
Rogers, J. et al. Clinical trial of indomethacin in Alzheimer's disease. Neurology 43, 1609–1611 (1993).
Scharf, S., Mander, A., Ugoni, A., Vajda, F. & Christophidis, V.N. A double-blind, placebo-controlled trial of diclofenac/misoprostol in Alzheimer's disease. Neurology 53, 197–201 (1999).
Hoozemans, J.J. & O'Banion, M.K. The role of COX-1 and COX-2 in Alzheimer's disease pathology and the therapeutic potentials of non-steroidal anti-inflammatory drugs. Curr. Drug Targets CNS Neurol. Disord. 4, 307–315 (2005).
Mackenzie, I.R.A. & Munoz, D.G. Nonsteroidal anti-inflammatory drug use and Alzheimer-type pathology in aging. Neurology 50, 986–990 (1998).
Lim, G.P. et al. Ibuprofen suppresses plaque pathology and inflammation in a mouse model for Alzheimer's disease. J. Neurosci. 20, 5709–5714 (2000).
Morgan, D., Gordon, M.N., Tan, J., Wilcock, D. & Rojiani, A.M. Dynamic complexity of the microglial activation response in transgenic models of amyloid deposition: implications for Alzheimer therapeutics. J. Neuropathol. Exp. Neurol. 64, 743–753 (2005).
Quinn, J. et al. Inflammation and cerebral amyloidosis are disconnected in an animal model of Alzheimer's disease. J. Neuroimmunol. 137, 32–41 (2003).
Lanz, T.A., Fici, G.J. & Merchant, K.M. Lack of specific amyloid-β(1–42) suppression by nonsteroidal anti-inflammatory drugs in young, plaque-free Tg2576 mice and in guinea pig neuronal cultures. J. Pharmacol. Exp. Ther. 312, 399–406 (2005).
Zhou, Y. et al. Nonsteroidal anti-inflammatory drugs can lower amyloidogenic Aβ42 by inhibiting Rho. Science 302, 1215–1217 (2003).
Tegeder, I., Pfeilschifter, J. & Geisslinger, G. Cyclooxygenase-independent actions of cyclooxygenase inhibitors. FASEB J. 15, 2057–2072 (2001).
Eriksen, J.L. et al. NSAIDs and enantiomers of flurbiprofen target γ-secretase and lower Aβ42 in vivo. J. Clin. Invest. 112, 440–449 (2003).
Sastre, M. et al. Nonsteroidal anti-inflammatory drugs repress β-secretase gene promoter activity by the activation of PPARγ. Proc. Natl. Acad. Sci. USA 103, 443–448 (2006).
Yan, Q. et al. Anti-inflammatory drug therapy alters beta-amyloid processing and deposition in an animal model of Alzheimer's disease. J. Neurosci. 23, 7504–7509 (2003).
Weggen, S. et al. A subset of NSAIDs lower amyloidogenic Aβ42 independently of cyclooxygenase activity. Nature 414, 212–216 (2001).
Takahashi, Y. et al. Sulindac sulfide is a noncompetitive γ-secretase inhibitor that preferentially reduces Aβ42 generation. J. Biol. Chem. 278, 18664–18670 (2003).
Lleo, A. et al. Nonsteroidal anti-inflammatory drugs lower Aβ42 and change presenilin 1 conformation. Nat. Med. 10, 1065–1066 (2004).
Beher, D. et al. Selected non-steroidal anti-inflammatory drugs and their derivatives target γ-secretase at a novel site. Evidence for an allosteric mechanism. J. Biol. Chem. 279, 43419–43426 (2004).
Gasparini, L., Rusconi, L., Xu, H., del Soldato, P. & Ongini, E. Modulation of beta-amyloid metabolism by non-steroidal anti-inflammatory drugs in neuronal cell cultures. J. Neurochem. 88, 337–348 (2004).
Kukar, T. et al. Diverse compounds mimic Alzheimer disease–causing mutations by augmenting Aβ42 production. Nat. Med. 11, 545–550 (2005).
Wyss-Coray, T. Killing pain, killing neurons? Nat. Med. 11, 472–473 (2005).
Tegeder, I. et al. Inhibition of NF-κB and AP-1 activation by R- and S-flurbiprofen. FASEB J. 15, 595–597 (2001).
Sung, S. et al. Modulation of nuclear factor-κB activity by indomethacin influences Aβ levels but not Aβ precursor protein metabolism in a model of Alzheimer's disease. Am. J. Pathol. 165, 2197–2206 (2004).
Monsonego, A. & Weiner, H.L. Immunotherapeutic approaches to Alzheimer's disease. Science 302, 834–838 (2003).
Games, D. et al. Alzheimer-type neuropathology in transgenic mice overexpressing V717F β-amyloid precursor protein. Nature 373, 523–527 (1995).
Hsia, A. et al. Plaque-independent disruption of neural circuits in Alzheimer's disease mouse models. Proc. Natl. Acad. Sci. USA 96, 3228–3233 (1999).
Masliah, E. et al. Comparison of neurodegenerative pathology in transgenic mice overexpressing V717F β-amyloid precursor protein and Alzheimer's disease. J. Neurosci. 16, 5795–5811 (1996).
Hsiao, K. et al. Correlative memory deficits, Aβ elevation, and amyloid plaques in transgenic mice. Science 274, 99–102 (1996).
Lewis, J. et al. Neurofibrillary tangles, amyotrophy and progressive motor disturbance in mice expressing mutant (P301L) tau protein. Nat. Genet. 25, 402–405 (2000).
McGowan, E., Eriksen, J. & Hutton, M. A decade of modeling Alzheimer's disease in transgenic mice. Trends Genet. 22, 281–289 (2006).
Oddo, S. et al. Triple-transgenic model of Alzheimer's disease with plaques and tangles: intracellular Aβ and synaptic dysfunction. Neuron 39, 409–421 (2003).
Wu, Z.L. et al. Comparative analysis of cortical gene expression in mouse models of Alzheimer's disease. Neurobiol. Aging 27, 377–386 (2006).
Dickey, C.A. et al. Selectively reduced expression of synaptic plasticity-related genes in amyloid precursor protein + presenilin-1 transgenic mice. J. Neurosci. 23, 5219–5226 (2003).
Reddy, P.H. et al. Gene expression profiles of transcripts in amyloid precursor protein transgenic mice: up-regulation of mitochondrial metabolism and apoptotic genes is an early cellular change in Alzheimer's disease. Hum. Mol. Genet. 13, 1225–1240 (2004).
Miller, R.J. & Tran, P.B. Chemokinetics. Neuron 47, 621–623 (2005).
Bellucci, A. et al. Induction of inflammatory mediators and microglial activation in mice transgenic for mutant human P301S tau protein. Am. J. Pathol. 165, 1643–1652 (2004).
Ho, L. et al. Gene expression profiling of the tau mutant (P301L) transgenic mouse brain. Neurosci. Lett. 310, 1–4 (2001).
Heneka, M.T. et al. Focal glial activation coincides with increased BACE1 activation and precedes amyloid plaque deposition in APP[V717I] transgenic mice. J. Neuroinflammation [online] 2, 22 (2005) (doi:10.1186/1742-2094-2-22).
Nagasaka, Y. et al. A unique gene expression signature discriminates familial Alzheimer's disease mutation carriers from their wild-type siblings. Proc. Natl. Acad. Sci. USA 102, 14854–14859 (2005).
Andreasson, K.I. et al. Age-dependent cognitive deficits and neuronal apoptosis in cyclooxygenase-2 transgenic mice. J. Neurosci. 21, 8198–8209 (2001).
Xiang, Z. et al. Cyclooxygenase-2 promotes amyloid plaque deposition in a mouse model of Alzheimer's disease neuropathology. Gene Expr. 10, 271–278 (2002).
Liang, X. et al. Deletion of the prostaglandin E2 EP2 receptor reduces oxidative damage and amyloid burden in a model of Alzheimer's disease. J. Neurosci. 25, 10180–10187 (2005).
Shie, F.S., Montine, K.S., Breyer, R.M. & Montine, T.J. Microglial EP2 as a new target to increase amyloid beta phagocytosis and decrease amyloid beta-induced damage to neurons. Brain Pathol. 15, 134–138 (2005).
Carroll, M.C. The role of complement and complement receptors in induction and regulation of immunity. Annu. Rev. Immunol. 16, 545–568 (1998).
Eikelenboom, P., Hack, C.E., Rozemuller, J.M. & Stam, F.C. Complement activation in amyloid plaques in Alzheimer's dementia. Virchows Arch. B Cell Pathol. Incl. Mol. Pathol. 56, 259–262 (1989).
Wyss-Coray, T. et al. Prominent neurodegeneration and increased plaque formation in complement-inhibited Alzheimer's mice. Proc. Natl. Acad. Sci. USA 99, 10837–10842 (2002).
Fonseca, M.I., Zhou, J., Botto, M. & Tenner, A.J. Absence of C1q leads to less neuropathology in transgenic mouse models of Alzheimer's disease. J. Neurosci. 24, 6457–6465 (2004).
Wyss-Coray, T. et al. TGF-β1 promotes microglial amyloid-β clearance and reduces plaque burden in transgenic mice. Nat. Med. 7, 612–618 (2001).
Wyss-Coray, T. et al. Adult mouse astrocytes degrade amyloid-β in vitro and in situ. Nat. Med. 9, 453–457 (2003).
Wyss-Coray, T., Lin, C., Sanan, D., Mucke, L. & Masliah, E. Chronic overproduction of TGF-β1 in astrocytes promotes Alzheimer's disease-like microvascular degeneration in transgenic mice. Am. J. Pathol. 156, 139–150 (2000).
Brionne, T.C., Tesseur, I., Masliah, E. & Wyss-Coray, T. Loss of TGF-β1 leads to increased neuronal cell death and microgliosis in mouse brain. Neuron 40, 1133–1145 (2003).
Weller, R.O. et al. Cerebral amyloid angiopathy. Amyloid β accumulates in putative interstitial fluid drainage pathways in Alzheimer's disease. Am. J. Pathol. 153, 725–733 (1998).
Tian, J., Shi, J., Bailey, K. & Mann, D.M. Negative association between amyloid plaques and cerebral amyloid angiopathy in Alzheimer's disease. Neurosci. Lett. 352, 137–140 (2003).
Yamamoto, M. et al. Overexpression of monocyte chemotactic protein-1/CCL2 in beta-amyloid precursor protein transgenic mice show accelerated diffuse beta-amyloid deposition. Am. J. Pathol. 166, 1475–1485 (2005).
Tan, J. et al. Role of CD40 ligand in amyloidosis in transgenic Alzheimer's mice. Nat. Neurosci. 5, 1288–1293 (2002).
Wyss-Coray, T. et al. Key signaling pathways regulate the biological activities and accumulation of amyloid-β. Neurobiol. Aging 22, 967–973 (2001).
Arancio, O. et al. RAGE potentiates Aβ-induced perturbation of neuronal function in transgenic mice. EMBO J. 23, 4096–4105 (2004).
Abraham, C.R., Shirahama, T. & Potter, H. Alpha 1-antichymotrypsin is associated solely with amyloid deposits containing the beta-protein. Amyloid and cell localization of alpha 1-antichymotrypsin. Neurobiol. Aging 11, 123–129 (1990).
Nilsson, L.N. et al. α-1-Antichymotrypsin promotes β-sheet amyloid plaque deposition in a transgenic mouse model of Alzheimer's disease. J. Neurosci. 21, 1444–1451 (2001).
Mucke, L. et al. Astroglial expression of human α1-antichymotrypsin enhances Alzheimer-like pathology in amyloid protein precursor transgenic mice. Am. J. Pathol. 157, 2003–2010 (2000).
Craft, J.M., Watterson, D.M., Frautschy, S.A. & Van Eldik, L.J. Aminopyridazines inhibit beta-amyloid-induced glial activation and neuronal damage in vivo. Neurobiol. Aging 25, 1283–1292 (2004).
Li, Y., Liu, L., Barger, S.W. & Griffin, W.S. Interleukin-1 mediates pathological effects of microglia on tau phosphorylation and on synaptophysin synthesis in cortical neurons through a p38-MAPK pathway. J. Neurosci. 23, 1605–1611 (2003).
Sheng, J.G., Zhu, S.G., Jones, R.A., Griffin, W.S.T. & Mrak, R.E. Interleukin-1 promotes expression and phosphorylation of neurofilament and tau proteins in vivo. Exp. Neurol. 163, 388–391 (2000).
Kitazawa, M., Oddo, S., Yamasaki, T.R., Green, K.N. & LaFerla, F.M. Lipopolysaccharide-induced inflammation exacerbates tau pathology by a cyclin-dependent kinase 5-mediated pathway in a transgenic model of Alzheimer's disease. J. Neurosci. 25, 8843–8853 (2005).
Schneider, A. et al. Hyperphosphorylation and aggregation of tau in experimental autoimmune encephalomyelitis. J. Biol. Chem. 279, 55833–55839 (2004).
Wegiel, J. et al. Reduced number and altered morphology of microglial cells in colony stimulating factor-1-deficient osteopetrotic op/op mice. Brain Res. 804, 135–139 (1998).
Kaku, M. et al. Amyloid beta protein deposition and neuron loss in osteopetrotic (op/op) mice. Brain Res. Brain Res. Protoc. 12, 104–108 (2003).
Hickey, W.F. Basic principles of immunological surveillance of the normal central nervous system. Glia 36, 118–124 (2001).
Ono, K. et al. Migration of exogenous immature hematopoietic cells into adult mouse brain parenchyma under GFP-expressing bone marrow chimera. Biochem. Biophys. Res. Commun. 262, 610–614 (1999).
Beck, H. et al. Participation of bone marrow-derived cells in long-term repair processes after experimental stroke. J. Cereb. Blood Flow Metab. 23, 709–717 (2003).
Malm, T.M. et al. Bone-marrow-derived cells contribute to the recruitment of microglial cells in response to beta-amyloid deposition in APP/PS1 double transgenic Alzheimer mice. Neurobiol. Dis. 18, 134–142 (2005).
Stalder, A.K. et al. Invasion of hematopoietic cells into the brain of amyloid precursor protein transgenic mice. J. Neurosci. 25, 11125–11132 (2005).
Simard, A.R., Soulet, D., Gowing, G., Julien, J.P. & Rivest, S. Bone marrow-derived microglia play a critical role in restricting senile plaque formation in Alzheimer's disease. Neuron 49, 489–502 (2006).
Chiang, C.S., McBride, W.H. & Withers, H.R. Radiation-induced astrocytic and microglial responses in mouse brain. Radiother. Oncol. 29, 60–68 (1993).
Fiala, M., et al. Ineffective phagocytosis of amyloid-beta by macrophages of Alzheimer's disease patients. J. Alzheimers Dis. 7, 221–232; discussion 255–262 (2005).
Togo, T. et al. Occurrence of T cells in the brain of Alzheimer's disease and other neurological diseases. J. Neuroimmunol. 124, 83–92 (2002).
Itagaki, S., McGeer, P.L. & Akiyama, H. Presence of T-cytotoxic suppressor and leucocyte common antigen positive cells in Alzheimer's disease brain tissue. Neurosci. Lett. 91, 259–264 (1988).
Rogers, J., Luber-Narod, J., Styren, S.D. & Civin, W.H. Expression of immune system-associated antigens by cells of the human central nervous system: relationship to the pathology of Alzheimer's disease. Neurobiol. Aging 9, 339–349 (1988).
Skias, D., Bania, M., Reder, A.T., Luchins, D. & Antel, J.P. Senile dementia of Alzheimer's type (SDAT): reduced T8+-cell-mediated suppressor activity. Neurology 35, 1635–1638 (1985).
Pirttila, T., Mattinen, S. & Frey, H. The decrease of CD8-positive lymphocytes in Alzheimer's disease. J. Neurol. Sci. 107, 160–165 (1992).
Richartz-Salzburger, E., et al. Altered lymphocyte distribution in Alzheimer's disease. J. Psychiatr. Res. advance online publication, 3 March 2006 (doi:10.1016/j.jpsychires.2006.01.010).
Dysken, M.W. et al. Distribution of peripheral lymphocytes in Alzheimer patients and controls. J. Psychiatr. Res. 26, 213–218 (1992).
Trieb, K., Ransmayr, G., Sgonc, R., Lassmann, H. & Grubeck-Loebenstein, B. APP peptides stimulate lymphocyte proliferation in normals, but not in patients with Alzheimer's disease. Neurobiol. Aging 17, 541–547 (1996).
Monsonego, A. et al. Increased T cell reactivity to amyloid beta protein in older humans and patients with Alzheimer disease. J. Clin. Invest. 112, 415–422 (2003).
Nath, A. et al. Autoantibodies to amyloid beta-peptide (Aβ) are increased in Alzheimer's disease patients and Aβ antibodies can enhance Aβ neurotoxicity: implications for disease pathogenesis and vaccine development. Neuromolecular Med. 3, 29–39 (2003).
Weksler, M.E. et al. Patients with Alzheimer disease have lower levels of serum anti-amyloid peptide antibodies than healthy elderly individuals. Exp. Gerontol. 37, 943–948 (2002).
Du, Y. et al. Reduced levels of amyloid β-peptide antibody in Alzheimer's disease. Neurology 57, 801–805 (2001).
Hyman, B.T. et al. Autoantibodies to amyloid-beta in Alzheimer's disease. Ann. Neurol. 49, 808–810 (2001).
Mruthinti, S. et al. Autoimmunity in Alzheimer's disease: increased levels of circulating IgGs binding Aβ and RAGE peptides. Neurobiol. Aging 25, 1023–1032 (2004).
Moir, R.D. et al. Autoantibodies to redox-modified oligomeric Aβ are attenuated in the plasma of Alzheimer's disease patients. J. Biol. Chem. 280, 17458–17463 (2005).
Eddleston, M. & Mucke, L. Molecular profile of reactive astrocytes—implications for their role in neurologic disease. Neuroscience 54, 15–36 (1993).
Mennicken, F., Maki, R., de Souza, E.B. & Quirion, R. Chemokines and chemokine receptors in the CNS: a possible role in neuroinflammation and patterning. Trends Pharmacol. Sci. 20, 73–78 (1999).
Gasque, P., Dean, Y.D., McGreal, E.P., Beek, J.V. & Morgan, B.P. Complement components of the innate immune system in health and disease in the CNS. Immunopharmacology 49, 171–186 (2000).
Warner, T.D. & Mitchell, J.A. Cyclooxygenases: new forms, new inhibitors, and lessons from the clinic. FASEB J. 18, 790–804 (2004).
Lehmann, J.M., Lenhard, J.M., Oliver, B.B., Ringold, G.M. & Kliewer, S.A. Peroxisome proliferator-activated receptors α and γ are activated by indomethacin and other non-steroidal anti-inflammatory drugs. J. Biol. Chem. 272, 3406–3410 (1997).
Bernardo, A., Ajmone-Cat, M.A., Gasparini, L., Ongini, E. & Minghetti, L. Nuclear receptor peroxisome proliferator-activated receptor-γ is activated in rat microglial cells by the anti-inflammatory drug HCT1026, a derivative of flurbiprofen. J. Neurochem. 92, 895–903 (2005).
Stellwagen, D. & Malenka, R.C. Synaptic scaling mediated by glial TNF-α. Nature 440, 1054–1059 (2006).
Wahl, S.M. Transforming growth factor beta (TGF-β) in inflammation: a cause and a cure. J. Clin. Immunol. 12, 61–74 (1992).
Acknowledgements
I would like to thank M. Buckwalter and M. Britschgi for comments on the manuscript, L. Bertram for advice on the use of the AlzGene genetic resource, and O. Arancio and M. Staufenbiel for communicating unpublished data. This work was supported by the John Douglas French Alzheimer's Foundation and the Veterans Administration Geriatric Research, Education and Clinical Center.
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The author declares no competing financial interests.
Rights and permissions
About this article
Cite this article
Wyss-Coray, T. Inflammation in Alzheimer disease: driving force, bystander or beneficial response?. Nat Med 12, 1005–1015 (2006). https://doi.org/10.1038/nm1484
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm1484
This article is cited by
-
Microglia at the Centre of Brain Research: Accomplishments and Challenges for the Future
Neurochemical Research (2022)
-
A New Approach to Model Sporadic Alzheimer’s Disease by Intracerebroventricular Streptozotocin Injection in APP/PS1 Mice
Molecular Neurobiology (2021)
-
Immunoinflammatory Aspects of Parkinson’s Disease
Neuroscience and Behavioral Physiology (2020)
-
The Promises and Challenges of Erythropoietin for Treatment of Alzheimer’s Disease
NeuroMolecular Medicine (2019)
-
Trans-cinnamaldehyde Modulates Hippocampal Nrf2 Factor and Inhibits Amyloid Beta Aggregation in LPS-Induced Neuroinflammation Mouse Model
Neurochemical Research (2018)