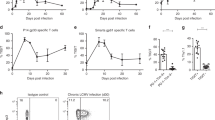
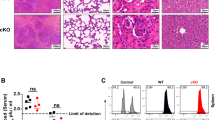
Abstract
Persistent viral infections are a major health concern. One obstacle inhibiting the clearance of persistent infections is functional inactivation of antiviral T cells. Although such immunosuppression occurs rapidly after infection, the mechanisms that induce the loss of T-cell activity and promote viral persistence are unknown. Herein we document that persistent viral infection in mice results in a significant upregulation of interleukin (IL)-10 by antigen-presenting cells, leading to impaired T-cell responses. Genetic removal of Il10 resulted in the maintenance of robust effector T-cell responses, the rapid elimination of virus and the development of antiviral memory T-cell responses. Therapeutic administration of an antibody that blocks the IL-10 receptor restored T-cell function and eliminated viral infection. Thus, we identify a single molecule that directly induces immunosuppression leading to viral persistence and demonstrate that a therapy to neutralize IL-10 results in T-cell recovery and the prevention of viral persistence.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Brooks, D.G., Teyton, L., Oldstone, M.B. & McGavern, D.B. Intrinsic functional dysregulation of CD4 T cells occurs rapidly following persistent viral infection. J. Virol. 79, 10514–10527 (2005).
Gallimore, A. et al. Induction and exhaustion of lymphocytic choriomeningitis virus-specific cytotoxic T lymphocytes visualized using soluble tetrameric major histocompatibility complex class I-peptide complexes. J. Exp. Med. 187, 1383–1393 (1998).
Lechner, F. et al. Analysis of successful immune responses in persons infected with hepatitis C virus. J. Exp. Med. 191, 1499–1512 (2000).
Rosenberg, E.S. et al. Immune control of HIV-1 after early treatment of acute infection. Nature 407, 523–526 (2000).
Thimme, R. et al. Determinants of viral clearance and persistence during acute hepatitis C virus infection. J. Exp. Med. 194, 1395–1406 (2001).
Zajac, A.J. et al. Viral immune evasion due to persistence of activated T cells without effector function. J. Exp. Med. 188, 2205–2213 (1998).
Ou, R., Zhou, S., Huang, L. & Moskophidis, D. Critical role for alpha/beta and gamma interferons in persistence of lymphocytic choriomeningitis virus by clonal exhaustion of cytotoxic T cells. J. Virol. 75, 8407–8423 (2001).
van der Most, R.G. et al. Identification of Db- and Kb-restricted subdominant cytotoxic T-cell responses in lymphocytic choriomeningitis virus-infected mice. Virology 240, 158–167 (1998).
Wherry, E.J., Blattman, J.N., Murali-Krishna, K., van der Most, R. & Ahmed, R. Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J. Virol. 77, 4911–4927 (2003).
Klenerman, P. & Hill, A. T cells and viral persistence: lessons from diverse infections. Nat. Immunol. 6, 873–879 (2005).
Barber, D.L. et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature 439, 682–687 (2005).
Borrow, P. & Oldstone, M.B. Lymphocytic choriomeningitis virus. In Viral Pathogenesis (ed. Nathanson, N.) 593–627 (Lippincott-Raven, Philadelphia, 1997).
Ahmed, R., Salmi, A., Butler, L.D., Chiller, J.M. & Oldstone, M.B. Selection of genetic variants of lymphocytic choriomeningitis virus in spleens of persistently infected mice. Role in suppression of cytotoxic T lymphocyte response and viral persistence. J. Exp. Med. 160, 521–540 (1984).
Borrow, P., Evans, C.F. & Oldstone, M.B. Virus-induced immunosuppression: immune system-mediated destruction of virus-infected dendritic cells results in generalized immune suppression. J. Virol. 69, 1059–1070 (1995).
Salvato, M., Borrow, P., Shimomaye, E. & Oldstone, M.B. Molecular basis of viral persistence: a single amino acid change in the glycoprotein of lymphocytic choriomeningitis virus is associated with suppression of the antiviral cytotoxic T-lymphocyte response and establishment of persistence. J. Virol. 65, 1863–1869 (1991).
Sevilla, N. et al. Immunosuppression and resultant viral persistence by specific viral targeting of dendritic cells. J. Exp. Med. 192, 1249–1260 (2000).
Fuller, M.J., Khanolkar, A., Tebo, A.E. & Zajac, A.J. Maintenance, loss, and resurgence of T cell responses during acute, protracted, and chronic viral infections. J. Immunol. 172, 4204–4214 (2004).
Fiorentino, D.F. et al. IL-10 acts on the antigen-presenting cell to inhibit cytokine production by Th1 cells. J. Immunol. 146, 3444–3451 (1991).
Groux, H., Bigler, M., de Vries, J.E. & Roncarolo, M.G. Interleukin-10 induces a long-term antigen-specific anergic state in human CD4+ T cells. J. Exp. Med. 184, 19–29 (1996).
Moore, K.W., de Waal Malefyt, R., Coffman, R.L. & O'Garra, A. Interleukin-10 and the interleukin-10 receptor. Annu. Rev. Immunol. 19, 683–765 (2001).
Clerici, M. et al. Human immunodeficiency virus (HIV) phenotype and interleukin-2/ interleukin-10 ratio are associated markers of protection and progression in HIV infection. Blood 88, 574–579 (1996).
Orsilles, M.A., Pieri, E., Cooke, P. & Caula, C. IL-2 and IL-10 serum levels in HIV-1-infected patients with or without active antiretroviral therapy. APMIS 114, 55–60 (2006).
Cacciarelli, T.V., Martinez, O.M., Gish, R.G., Villanueva, J.C. & Krams, S.M. Immunoregulatory cytokines in chronic hepatitis C virus infection: pre- and posttreatment with interferon alfa. Hepatology 24, 6–9 (1996).
Brady, M.T., MacDonald, A.J., Rowan, A.G. & Mills, K.H. Hepatitis C virus non-structural protein 4 suppresses Th1 responses by stimulating IL-10 production from monocytes. Eur. J. Immunol. 33, 3448–3457 (2003).
Dolganiuc, A. et al. Hepatitis C virus core and nonstructural protein 3 proteins induce pro- and anti-inflammatory cytokines and inhibit dendritic cell differentiation. J. Immunol. 170, 5615–5624 (2003).
Marin-Serrano, E., Rodriguez-Ramos, C., Diaz, F., Martin-Herrera, L. & Giron-Gonzalez, J.A. Modulation of the anti-inflammatory interleukin 10 and of proapoptotic IL-18 in patients with chronic hepatitis C treated with interferon alpha and ribavirin. J. Viral Hepat. 13, 230–234 (2006).
Rico, M.A. et al. Hepatitis B virus-specific T-cell proliferation and cytokine secretion in chronic hepatitis B e antibody-positive patients treated with ribavirin and interferon alpha. Hepatology 33, 295–300 (2001).
Clerici, M. et al. Role of interleukin-10 in T helper cell dysfunction in asymptomatic individuals infected with the human immunodeficiency virus. J. Clin. Invest. 93, 768–775 (1994).
Landay, A.L. et al. In vitro restoration of T cell immune function in human immunodeficiency virus-positive persons: effects of interleukin (IL)-12 and anti-IL-10. J. Infect. Dis. 173, 1085–1091 (1996).
Rigopoulou, E.I., Abbott, W.G., Haigh, P. & Naoumov, N.V. Blocking of interleukin-10 receptor–a novel approach to stimulate T-helper cell type 1 responses to hepatitis C virus. Clin. Immunol. 117, 57–64 (2005).
Lin, M.T., Hinton, D.R., Parra, B., Stohlman, S.A. & van der Veen, R.C. The role of IL-10 in mouse hepatitis virus-induced demyelinating encephalomyelitis. Virology 245, 270–280 (1998).
Strestik, B.D., Olbrich, A.R., Hasenkrug, K.J. & Dittmer, U. The role of IL-5, IL-6 and IL-10 in primary and vaccine-primed immune responses to infection with Friend retrovirus (Murine leukaemia virus). J. Gen. Virol. 82, 1349–1354 (2001).
Day, C.L. et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature 443, 350–354 (2006).
Trautmann, L. et al. Upregulation of PD-1 expression on HIV-specific CD8 + T cells leads to reversible immune dysfunction. Nat. Med. advance online publication, 20 August 2006 (doi:10.1038/nm1482).
Carbonneil, C., Donkova-Petrini, V., Aouba, A. & Weiss, L. Defective dendritic cell function in HIV-infected patients receiving effective highly active antiretroviral therapy: neutralization of IL-10 production and depletion of CD4+CD25+ T cells restore high levels of HIV-specific CD4+ T cell responses induced by dendritic cells generated in the presence of IFN–α. J. Immunol. 172, 7832–7840 (2004).
Granelli-Piperno, A., Golebiowska, A., Trumpfheller, C., Siegal, F.P. & Steinman, R.M. HIV-1-infected monocyte-derived dendritic cells do not undergo maturation but can elicit IL-10 production and T cell regulation. Proc. Natl. Acad. Sci. USA 101, 7669–7674 (2004).
Ji, J., Sahu, G.K., Braciale, V.L. & Cloyd, M.W. HIV-1 induces IL-10 production in human monocytes via a CD4-independent pathway. Int. Immunol. 17, 729–736 (2005).
Kinter, A.L. et al. CD25+CD4+ regulatory T cells from the peripheral blood of asymptomatic HIV-infected individuals regulate CD4+ and CD8+ HIV-specific T cell immune responses in vitro and are associated with favorable clinical markers of disease status. J. Exp. Med. 200, 331–343 (2004).
Lechmann, M. et al. Decreased frequency of HCV core-specific peripheral blood mononuclear cells with type 1 cytokine secretion in chronic hepatitis C. J. Hepatol. 31, 971–978 (1999).
Ostrowski, M.A. et al. Quantitative and qualitative assessment of human immunodeficiency virus type 1 (HIV-1)-specific CD4+ T cell immunity to gag in HIV-1-infected individuals with differential disease progression: reciprocal interferon-γ and interleukin-10 responses. J. Infect. Dis. 184, 1268–1278 (2001).
Tsai, S.L., Liaw, Y.F., Chen, M.H., Huang, C.Y. & Kuo, G.C. Detection of type 2-like T-helper cells in hepatitis C virus infection: implications for hepatitis C virus chronicity. Hepatology 25, 449–458 (1997).
Battegay, M. et al. Enhanced establishment of a virus carrier state in adult CD4+ T-cell-deficient mice. J. Virol. 68, 4700–4704 (1994).
Matloubian, M., Concepcion, R.J. & Ahmed, R. CD4+ T cells are required to sustain CD8+ cytotoxic T-cell responses during chronic viral infection. J. Virol. 68, 8056–8063 (1994).
Tishon, A., Lewicki, H., Rall, G., Von Herrath, M. & Oldstone, M.B. An essential role for type 1 interferon-γ in terminating persistent viral infection. Virology 212, 244–250 (1995).
Brooks, D.G., McGavern, D.B. & Oldstone, M.B. Reprogramming of antiviral T cells prevents inactivation and restores T cell activity during persistent viral infection. J. Clin. Invest. 116, 1675–1685 (2006).
Accapezzato, D. et al. Hepatic expansion of a virus-specific regulatory CD8+ T cell population in chronic hepatitis C virus infection. J. Clin. Invest. 113, 963–972 (2004).
Homann, D., Teyton, L. & Oldstone, M.B. Differential regulation of antiviral T-cell immunity results in stable CD8+ but declining CD4+ T-cell memory. Nat. Med. 7, 913–919 (2001).
Zuniga, E.I., McGavern, D.B., Pruneda-Paz, J.L., Teng, C. & Oldstone, M.B. Bone marrow plasmacytoid dendritic cells can differentiate into myeloid dendritic cells upon virus infection. Nat. Immunol. 5, 1227–1234 (2004).
Acknowledgements
We thank H. Lewicki, D. Young and J. Wheatley for technical assistance and F. Chisari for analysis of sALT levels. Independent findings of IL-10R blockade controlling persistent infection were also found by Ejrneas et al. (JEM, in press). Our work was supported by NIH training grant AI07244-22 (D.G.B.), NIH grants AI09484, AI45927 (M.B.A.O.), AI062718-01 (D.B.M.), NS048866-01 (D.B.M.) and a Dana Foundation grant (D.B.M.). This is publication number 18209-MIND from the Viral Immunobiology Laboratory, Department of Molecular and Integrative Neuroscience.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
Increased IL-10 production by CD4 T cells early during Cl 13 infection. (PDF 275 kb)
Supplementary Fig. 2
Expression of IL-10 receptor during LCMV infection. (PDF 307 kb)
Supplementary Fig. 3
PD-1 and Foxp3 expression by T cells during LCMV infection. (PDF 465 kb)
Rights and permissions
About this article
Cite this article
Brooks, D., Trifilo, M., Edelmann, K. et al. Interleukin-10 determines viral clearance or persistence in vivo. Nat Med 12, 1301–1309 (2006). https://doi.org/10.1038/nm1492
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm1492
This article is cited by
-
Aspirin inhibits rotavirus replication and alters rat gut microbial composition
Virology Journal (2023)
-
Chronic LCMV infection regulates the effector T cell response by inducing the generation of less immunogenic dendritic cells
Experimental & Molecular Medicine (2023)
-
Regulation of CD8+ T memory and exhaustion by the mTOR signals
Cellular & Molecular Immunology (2023)
-
Impact of Mycobacterium avium subsp. paratuberculosis infection on bovine IL10RA knockout mammary epithelial (MAC-T) cells
In Vitro Cellular & Developmental Biology - Animal (2023)
-
Development and external evaluation of predictions models for mortality of COVID-19 patients using machine learning method
Neural Computing and Applications (2023)