Abstract
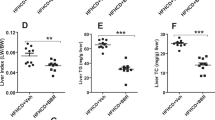
We identify berberine (BBR), a compound isolated from a Chinese herb, as a new cholesterol-lowering drug. Oral administration of BBR in 32 hypercholesterolemic patients for 3 months reduced serum cholesterol by 29%, triglycerides by 35% and LDL-cholesterol by 25%. Treatment of hyperlipidemic hamsters with BBR reduced serum cholesterol by 40% and LDL-cholesterol by 42%, with a 3.5-fold increase in hepatic LDLR mRNA and a 2.6-fold increase in hepatic LDLR protein. Using human hepatoma cells, we show that BBR upregulates LDLR expression independent of sterol regulatory element binding proteins, but dependent on ERK activation. BBR elevates LDLR expression through a post-transcriptional mechanism that stabilizes the mRNA. Using a heterologous system with luciferase as a reporter, we further identify the 5′ proximal section of the LDLR mRNA 3′ untranslated region responsible for the regulatory effect of BBR. These findings show BBR as a new hypolipidemic drug with a mechanism of action different from that of statin drugs.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Brown, M.S. & Goldstein, J.L. A receptor-mediated pathway for cholesterol homeostasis. Science 232, 34–47 (1986).
Goldstein, J.L. & Brown, M.S. Regulation of the mevalonate pathway. Nature 343, 425–430 (1990).
Grundy, S.M. Statin trials and goals of cholesterol-lowering therapy. Circulation 97, 1436–1439 (1998).
Ansell, B.J., Watson, K.E. & Fogelman, A.M. An evidence-based assessment of the NCEP adult treatment panel II guidelines. National cholesterol education program. JAMA 282, 2051–2057 (1999).
Smith, J.R., Osborne, T.F.G.J.L. & Brown, M.S. Identification of nucleotides responsible for enhancer activity of sterol regulatory element in low density lipoprotein receptor gene. J. Biol. Chem. 265, 2306–2310 (1990).
Briggs, M.R., Yokoyama, C., Wang, X.B.M.S. & Goldstein, J.L. Nuclear protein that binds sterol regulatory element of low density lipoprotein receptor promoter. J. Biol. Chem. 268, 14490–14496 (1993).
Wang, X., Briggs, M.R., Yokoyama, C., Goldstein, J.L. & Brown, M.S. Nuclear protein that binds sterol regulatory element of low density lipoprotein receptor promoter II. Purification and characterization. J. Biol. Chem. 268, 14497–14504 (1993).
Yokoyama, C. et al. SREBP-1, a basic-helix-loop-helix-leucine zipper protein that controls transcription of the low density lipoprotein receptor gene. Cell 75, 187–197 (1993).
Wang, X., Sato, R., Brown, M.S., Hua, X. & Goldstein, J.L. SREBP-1, a membrane bound transcription factor released by sterol-regulated proteolysis. Cell 77, 53–62 (1994).
Norturfft, A., DeBose-Boyd, R.A., Scheek, S., Goldstein, J.L. & Brown, M.S. Sterols regulate cycling of SREBP cleavage-activating protein (SCAP) between endoplasmic reticulum and Golgi. Proc. Natl. Acad. Sci. USA 96, 11235–11240 (1999).
Brown, M.S. & Goldstein, J.L. A proteolytic pathway that controls the cholesterol content of membranes, cells, and blood. Proc. Natl. Acad. Sci. USA 96, 11041–11048 (1999).
Nohturfft, A., Yabe, D., Goldstein, J.L., Brown, M.S. & Espenshade, P.J. Regulated step in cholesterol feedback localized to budding of SCAP from ER membrane. Cell 102, 315–323 (2000).
Yang, T., Goldstein, J.L. & Brown, M.S. Overexpression of membrane domain of SCAP prevents sterols from inhibiting SCAP. SREBP exit from endoplasmic reticulum. J. Biol. Chem. 275, 29881–29886 (2000).
Sakai, J. & Rawson, R.B. The sterol regulatory element-protein pathway: control of lipid homeostasis through regulated intracellular transport. Curr. Opin. Lipidol. 12, 261–266 (2001).
Goldstein, J.L., Rawson, R.B. & Brown, M.S. Mutant mammalian cells as tools to delineate the sterol regulatory element-binding protein pathway for feedback regulation of lipid synthesis. Arch. Biochem. Biophys. 397, 139–148 (2002).
LaRosa, J.C. & He, J.V.S. Effect of statins on risk of coronary disease: a meta-analysis of randomized controlled trials. JAMA 282, 2340–2346 (1999).
MRC/BHF. Heart protection study of cholesterol lowering with simvastatin in 20536 high-risk individuals: a randomised placebo-controlled trial. Lancet 360, 7–22 (2002).
Yeung, A. & Tsao, P. Statin therapy: beyond cholesterol lowering and antiinflammatory effects. Circulation 105, 2937–2938 (2002).
Expert panel on detection evaluation and treatment of high blood cholesterol in adults. Executive summary of the third report of the national cholesterol education program (NCEP). JAMA 285, 2486–2497 (2001).
Cho, E. Berberini hydrochloride. in Pharmacopoeia of the People's Republic of China 2, 437–439 (1990).
Luo, L.J. Experience of berberine in the treatment of diarrhea. Chin. J. Med. 41, 452–455 (1955).
Lau, C.W., Yao, X.Q., Chen, Z.Y., Ko, W.H. & Huang, Y. Cardiovascular actions of berberine. Cardiovasc. Drug Rev. 19, 234–244 (2001).
Liu . Berberine. Altern. Med. Rev. 5, 175–177 (2000).
Grand-Perret, T. et al. SCAP ligands are potent new lipid-lowering drugs. Nat. Med. 7, 1332–1338 (2001).
Liu, J., Ahlborn, T.E., Briggs, M.R. & Kraemer, F.B. Identification of a novel sterol-independent regulatory element in the human low density lipoprotein receptor promoter. J. Biol. Chem. 275, 5214–5221 (2000).
Zhang, F., Ahlborn, T.E., Li, C., Kraemer, F.B. & Liu, J. Identification of Egr1 as the oncostatin M-induced transcription activator that binds to the sterol-independent regulatory element of the human LDL receptor promoter. J. Lipid Res. 43, 1477–1485 (2002).
Liu, J., Zhang, F., Li, C., Lin, M. & Briggs, M.R. Synergistic activation of human LDL receptor expression by SCAP ligand and cytokine oncostatin M. Arterioscler. Thromb. Vasc. Biol. 23, 90–96 (2003).
Yamamoto, T. et al. The human LDL receptor: A cyctein-rich protein with multiple Alu sequence in its mRNA. Cell 39, 27–38 (1984).
Knouff, C., Malloy, S., Wilder, J., Altenburg, M.K. & Maeda, N. Doubling expression of the low density lipoprotein receptor by truncation of the 3′-untranslated region sequence ameliorates type III hyperlipoproteinemia in mice expressing the human apoE2 isoform. J. Biol. Chem. 276, 3856–3862 (2001).
Wilson, G.M., Vasa, M.Z. & Deeley, R.G. Stabilization and cytoskeletal-association of LDL receptor mRNA are mediated by distinct domains in its 3′ untranslated region. J. Lipid Res. 39, 1025–1032 (1998).
Sugiura, R. et al. Feedback regulation of MAPK signaling by an RNA-binding protein. Nature 424, 961–965 (2003).
Zhao, S.P. Etiology and diagnosis of hyperlipidemia. Clinical Serum and Lipid 1, 72–94 (1997).
Horton, J.D., Cuthbert, J.A. & Spady, D.K. Dietary fatty acids regulate hepatic low-density lipoprotein (LDL) transport by altering LDL receptor protein and messenger-RNA levels. J. Clin. Invest. 92, 743–749 (1993).
Bensch, W.R., Gadski, R.A., Bean, J.S., et al. Effects of LY295427, a low-density lipoprotein (LDL) receptor up-regulator, on LDL receptor gene transcription and cholesterol metabolism in normal and hypercholesterolemic hamsters. J. Pharmacol. Exp. Ther. 289, 85–92 (1999).
Ugawa, T., Kakuta, H., Moritani, H. & Inagaki, O. Effect of YM-53601, a novel squalene synthase inhibitor, on the clearance rate of plasma LDL and VLDL in hamsters. Br. J. Pharmacol. 137, 561–567 (2002).
Nakahara, M., Fujii, H., Maloney, P.R., Shimizu, M. & Sato, R. Bile acids enhance low density lipoprotein receptor gene expression via a MAPK cascade-mediated stabilization of mRNA. J. Biol. Chem. 277, 37229–37234 (2002).
Liu, J. et al. Novel mechanism of transcriptional activation of hepatic LDL receptor by oncostatin M. J. Lipid Res. 38, 2035–2048 (1997).
Liu, J., Hadjokas, N., Mosley, B. & Vestal, R.E. Oncostatin M-specific receptor expression and function in regulating cell proliferation of normal and malignant mammary epithelial cells. Cytokine 10, 295–302 (1998).
Dixon, D.A., Kaplan, C.D., McIntyre, T.M., Zimmerman, G.A. & Prescott, S.M. Post-transcriptional control of cyclooxygenase-2 gene expression. J. Biol. Chem. 275, 11750–11757 (2000).
Acknowledgements
We thank F. B. Kraemer for his review of the manuscript and S. M. Prescott for providing the pLuc plasmid. This study was supported by the National Natural Sciences Foundation of China (39925037, 39870889 & 39930190; J.-D.J.), by the Department of Veterans Affairs (Office of Research and Development, Medical Research Service; J. Liu) and by grant (1RO1CA83648-01; J.L.) from United States National Institute of Health.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Table 1
Effects of BBR in the entire hypercholesterolemic patient cohort (PDF 25 kb)
Supplementary Table 2
Characterization of the subgroup of hypercholesterolemic patients who were not taking other medication before or during BBR treatment (PDF 16 kb)
Supplementary Table 3
Lipid-lowering effect of BBR in hypercholesterolemia Type IIa & IIb (PDF 12 kb)
Rights and permissions
About this article
Cite this article
Kong, W., Wei, J., Abidi, P. et al. Berberine is a novel cholesterol-lowering drug working through a unique mechanism distinct from statins. Nat Med 10, 1344–1351 (2004). https://doi.org/10.1038/nm1135
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm1135
This article is cited by
-
Bioactive materials from berberine-treated human bone marrow mesenchymal stem cells promote alveolar bone regeneration by regulating macrophage polarization
Science China Life Sciences (2024)
-
Neuroprotective effect of Berberine Nanoparticles Against Seizures in Pentylenetetrazole Induced Kindling Model of Epileptogenesis: Role of Anti-Oxidative, Anti-Inflammatory, and Anti-Apoptotic Mechanisms
Neurochemical Research (2023)
-
Berberine increases stromal production of Wnt molecules and activates Lgr5+ stem cells to promote epithelial restitution in experimental colitis
BMC Biology (2022)
-
Integrated lipidomics and network pharmacology analysis to reveal the mechanisms of berberine in the treatment of hyperlipidemia
Journal of Translational Medicine (2022)
-
Enhanced CHOLESTEROL biosynthesis promotes breast cancer metastasis via modulating CCDC25 expression and neutrophil extracellular traps formation
Scientific Reports (2022)