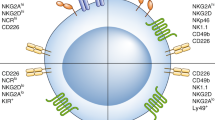
Abstract
Natural killer (NK) cells influence innate and adaptive immune host defenses. Existing data indicate that manipulating the balance between inhibitory and activating NK receptor signals, the sensitivity of target cells to NK cell-mediated apoptosis, and NK cell cross-talk with dendritic cells might hold therapeutic promise. Efforts to modulate NK cell trafficking into inflamed tissues and/or lymph nodes, and to counteract NK cell suppressors, might also prove fruitful in the clinic. However, deeper investigation into the benefits of combination therapy, greater understanding of the functional distinctions between NK cell subsets, and design of new tools to monitor NK cell activity are needed to strengthen our ability to harness the power of NK cells for therapeutic aims.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Herberman, R.B., Nunn, M.E., Holden, H.T. & Lavrin, D.H. Natural cytotoxic reactivity of mouse lymphoid cells against syngeneic and allogeneic tumors. II. Characterization of effector cells. Int. J. Cancer 16, 230–239 (1975).
Long, E.O. Regulation of immune responses through inhibitory receptors. Annu. Rev. Immunol. 17, 875–904 (1999).
Orange, J.S. & Ballas, Z.K. Natural killer cells in human health and disease. Clin. Immunol. 118, 1–10 (2006).
Moretta, L. & Moretta, A. Unravelling natural killer cell function: triggering and inhibitory human NK receptors. EMBO J. 23, 255–259 (2004).
Cerwenka, A. & Lanier, L.L. Ligands for natural killer cell receptors: redundancy or specificity. Immunol. Rev. 181, 158–169 (2001).
Hayakawa, Y., Huntington, N.D., Nutt, S.L. & Smyth, M.J. Functional subsets of mouse natural killer cells. Immunol. Rev. 214, 47–55 (2006).
Walzer, T. et al. Identification, activation, and selective in vivo ablation of mouse NK cells via NKp46. Proc. Natl. Acad. Sci. USA 104, 3384–3389 (2007).
Albertsson, P.A. et al. NK cells and the tumour microenvironment: implications for NK-cell function and anti-tumour activity. Trends Immunol. 24, 603–609 (2003).
Kim, S., Iizuka, K., Aguila, H.L., Weissman, I.L. & Yokoyama, W.M. In vivo natural killer cell activities revealed by natural killer cell-deficient mice. Proc. Natl. Acad. Sci. USA 97, 2731–2736 (2000).
Coca, S. et al. The prognostic significance of intratumoral natural killer cells in patients with colorectal carcinoma. Cancer 79, 2320–2328 (1997).
Chang, C.C., Campoli, M. & Ferrone, S. Classical and nonclassical HLA class I antigen and NK cell-activating ligand changes in malignant cells: current challenges and future directions. Adv. Cancer Res. 93, 189–234 (2005).
Gasser, S., Orsulic, S., Brown, E.J. & Raulet, D.H. The DNA damage pathway regulates innate immune system ligands of the NKG2D receptor. Nature 436, 1186–1190 (2005).
Diefenbach, A., Jensen, E.R., Jamieson, A.M. & Raulet, D.H. Rae1 and H60 ligands of the NKG2D receptor stimulate tumour immunity. Nature 413, 165–171 (2001).
Smyth, M.J. et al. NKG2D function protects the host from tumor initiation. J. Exp. Med. 202, 583–588 (2005).
Vetter, C.S., Lieb, W., Brocker, E.B. & Becker, J.C. Loss of nonclassical MHC molecules MIC-A/B expression during progression of uveal melanoma. Br. J. Cancer 91, 1495–1499 (2004).
Groh, V., Wu, J., Yee, C. & Spies, T. Tumour-derived soluble MIC ligands impair expression of NKG2D and T-cell activation. Nature 419, 734–738 (2002).
Imai, K., Matsuyama, S., Miyake, S., Suga, K. & Nakachi, K. Natural cytotoxic activity of peripheral-blood lymphocytes and cancer incidence: an 11-year follow-up study of a general population. Lancet 356, 1795–1799 (2000).
Hayashi, T. et al. Identification of the NKG2D haplotypes associated with natural cytotoxic activity of peripheral blood lymphocytes and cancer immunosurveillance. Cancer Res. 66, 563–570 (2006).
Ferlazzo, G. et al. Human dendritic cells activate resting natural killer (NK) cells and are recognized via the NKp30 receptor by activated NK cells. J. Exp. Med. 195, 343–351 (2002).
Sivori, S. et al. Involvement of natural cytotoxicity receptors in human natural killer cell-mediated lysis of neuroblastoma and glioblastoma cell lines. J. Neuroimmunol. 107, 220–225 (2000).
Fauriat, C. et al. Deficient expression of NCR in NK cells from acute myeloid leukemia: evolution during leukemia treatment and impact of leukemia cells in NCRdull phenotype induction. Blood 109, 323–330 (2007).
Carrington, M. et al. Hierarchy of resistance to cervical neoplasia mediated by combinations of killer immunoglobulin-like receptor and human leukocyte antigen loci. J. Exp. Med. 201, 1069–1075 (2005).
Lopez-Vazquez, A. et al. Protective effect of the HLA-Bw4I80 epitope and the killer cell immunoglobulin-like receptor 3DS1 gene against the development of hepatocellular carcinoma in patients with hepatitis C virus infection. J. Infect. Dis. 192, 162–165 (2005).
Borg, C. et al. Novel mode of action of c-kit tyrosine kinase inhibitors leading to NK cell-dependent antitumor effects. J. Clin. Invest. 114, 379–388 (2004).
Ghiringhelli, F., Menard, C., Martin, F. & Zitvogel, L. The role of regulatory T cells in the control of natural killer cells: relevance during tumor progression. Immunol. Rev. 214, 229–238 (2006).
Orange, J.S. Human natural killer cell deficiencies. Curr. Opin. Allergy Clin. Immunol. 6, 399–409 (2006).
Orange, J.S. Human natural killer cell deficiencies and susceptibility to infection. Microbes Infect. 4, 1545–1558 (2002).
Bashirova, A.A., Martin, M.P., McVicar, D.W. & Carrington, M. The killer immunoglobulin-like receptor gene cluster: tuning the genome for defense. Annu. Rev. Genomics Hum. Genet. 7, 277–300 (2006).
Martin, M.P. et al. Epistatic interaction between KIR3DS1 and HLA-B delays the progression to AIDS. Nat. Genet. 31, 429–434 (2002).
Khakoo, S.I. et al. HLA and NK cell inhibitory receptor genes in resolving hepatitis C virus infection. Science 305, 872–874 (2004).
Gumá, M. et al. Expansion of CD94/NKG2C+ NK cells in response to human cytomegalovirus-infected fibroblasts. Blood 107, 3624–3631 (2006).
Hiby, S.E. et al. Combinations of maternal KIR and fetal HLA-C genes influence the risk of preeclampsia and reproductive success. J. Exp. Med. 200, 957–965 (2004).
Hanna, J. et al. Decidual NK cells regulate key developmental processes at the human fetal-maternal interface. Nat. Med. 12, 1065–1074 (2006).
Liu, R. et al. Autoreactive T cells mediate NK cell degeneration in autoimmune disease. J. Immunol. 176, 5247–5254 (2006).
Zimmer, J., Bausinger, H. & de la Salle, H. Autoimmunity mediated by innate immune effector cells. Trends Immunol. 22, 300–301 (2001).
Vollmer, T. et al. Oral simvastatin treatment in relapsing-remitting multiple sclerosis. Lancet 363, 1607–1608 (2004).
Van Kaer, L. α-Galactosylceramide therapy for autoimmune diseases: prospects and obstacles. Nat. Rev. Immunol. 5, 31–42 (2005).
Bielekova, B. et al. Regulatory CD56bright natural killer cells mediate immunomodulatory effects of IL-2Rα-targeted therapy (daclizumab) in multiple sclerosis. Proc. Natl. Acad. Sci. USA 103, 5941–5946 (2006).
Grimm, E.A., Mazumder, A., Zhang, H.Z. & Rosenberg, S.A. Lymphokine-activated killer cell phenomenon. Lysis of natural killer-resistant fresh solid tumor cells by interleukin 2-activated autologous human peripheral blood lymphocytes. J. Exp. Med. 155, 1823–1841 (1982).
Rosenberg, S.A. et al. Observations on the systemic administration of autologous lymphokine-activated killer cells and recombinant interleukin-2 to patients with metastatic cancer. N. Engl. J. Med. 313, 1485–1492 (1985).
Law, T.M. et al. Phase III randomized trial of interleukin-2 with or without lymphokine-activated killer cells in the treatment of patients with advanced renal cell carcinoma. Cancer 76, 824–832 (1995).
Lotze, M.T. & Rosenberg, S.A. Results of clinical trials with the administration of interleukin 2 and adoptive immunotherapy with activated cells in patients with cancer. Immunobiology 172, 420–437 (1986).
Ueda, Y. et al. Clinical application of adoptive immunotherapy and IL-2 for the treatment of advanced digestive tract cancer. Hepatogastroenterology 46 (suppl. 1), 1274–1279 (1999).
Burns, L.J. et al. IL-2-based immunotherapy after autologous transplantation for lymphoma and breast cancer induces immune activation and cytokine release: a phase I/II trial. Bone Marrow Transplant. 32, 177–186 (2003).
Kammula, U.S., White, D.E. & Rosenberg, S.A. Trends in the safety of high dose bolus interleukin-2 administration in patients with metastatic cancer. Cancer 83, 797–805 (1998).
Ghiringhelli, F. et al. CD4+CD25+ regulatory T cells inhibit natural killer cell functions in a transforming growth factor–β–dependent manner. J. Exp. Med. 202, 1075–1085 (2005).
Rodella, L. et al. Interleukin 2 and interleukin 15 differentially predispose natural killer cells to apoptosis mediated by endothelial and tumour cells. Br. J. Haematol. 115, 442–450 (2001).
Smyth, M.J. et al. NKG2D recognition and perforin effector function mediate effective cytokine immunotherapy of cancer. J. Exp. Med. 200, 1325–1335 (2004).
Mortier, E. et al. Soluble interleukin-15 receptor α (IL-15Rα)-sushi as a selective and potent agonist of IL-15 action through IL-15Rβ/γ. Hyperagonist IL-15·IL-15Rα fusion proteins. J. Biol. Chem. 281, 1612–1619 (2006).
Ruggeri, L. et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science 295, 2097–2100 (2002).
Giebel, S. et al. Survival advantage with KIR ligand incompatibility in hematopoietic stem cell transplantation from unrelated donors. Blood 102, 814–819 (2003).
Miller, J.S. et al. Missing KIR ligands are associated with less relapse and increased graft-versus-host disease (GVHD) following unrelated donor allogeneic HCT. Blood 109, 5058–5061 (2007).
Davies, S.M. et al. Evaluation of KIR ligand incompatibility in mismatched unrelated donor hematopoietic transplants. Killer immunoglobulin-like receptor. Blood 100, 3825–3827 (2002).
Farag, S.S. et al. The effect of KIR ligand incompatibility on the outcome of unrelated donor transplantation: a report from the center for international blood and marrow transplant research, the European blood and marrow transplant registry, and the Dutch registry. Biol. Blood Marrow Transplant. 12, 876–884 (2006).
Aversa, F. et al. Treatment of high-risk acute leukemia with T-cell-depleted stem cells from related donors with one fully mismatched HLA haplotype. N. Engl. J. Med. 339, 1186–1193 (1998).
Cooley, S. et al. KIR reconstitution is altered by T cells in the graft and correlates with clinical outcomes after unrelated donor transplantation. Blood 106, 4370–4376 (2005).
Passweg, J.R., Stern, M., Koehl, U., Uharek, L. & Tichelli, A. Use of natural killer cells in hematopoetic stem cell transplantation. Bone Marrow Transplant. 35, 637–643 (2005).
Passweg, J.R. et al. Purified donor NK-lymphocyte infusion to consolidate engraftment after haploidentical stem cell transplantation. Leukemia 18, 1835–1838 (2004).
Miller, J.S. et al. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood 105, 3051–3057 (2005).
Klingemann, H.G. Natural killer cell-based immunotherapeutic strategies. Cytotherapy 7, 16–22 (2005).
Delahaye, N.F., Barbier, M., Fumoux, F. & Rihet, P. Association analyses of NCR3 polymorphisms with P. falciparum mild malaria. Microbes Infect. 9, 160–166 (2007).
Ljunggren, H.G. & Malmberg, K.J. Prospects for the use of NK cells in immunotherapy of human cancer. Nat. Rev. Immunol. 7, 329–339 (2007).
Bryceson, Y.T., March, M.E., Ljunggren, H.G. & Long, E.O. Synergy among receptors on resting NK cells for the activation of natural cytotoxicity and cytokine secretion. Blood 107, 159–166 (2006).
Koh, C.Y. et al. Augmentation of antitumor effects by NK cell inhibitory receptor blockade in vitro and in vivo. Blood 97, 3132–3137 (2001).
Sheridan, C. First-in-class cancer therapeutic to stimulate natural killer cells. Nat. Biotechnol. 24, 597 (2006).
Carter, P.J. Potent antibody therapeutics by design. Nat. Rev. Immunol. 6, 343–357 (2006).
Cartron, G. et al. Therapeutic activity of humanized anti-CD20 monoclonal antibody and polymorphism in IgG Fc receptor FcγRIIIa gene. Blood 99, 754–758 (2002).
Weng, W.K. & Levy, R. Two immunoglobulin G fragment C receptor polymorphisms independently predict response to rituximab in patients with follicular lymphoma. J. Clin. Oncol. 21, 3940–3947 (2003).
Khan, K.D. et al. A phase 2 study of rituximab in combination with recombinant interleukin-2 for rituximab-refractory indolent non-Hodgkin's lymphoma. Clin. Cancer Res. 12, 7046–7053 (2006).
Shahied, L.S. et al. Bispecific minibodies targeting HER2/neu and CD16 exhibit improved tumor lysis when placed in a divalent tumor antigen binding format. J. Biol. Chem. 279, 53907–53914 (2004).
Uherek, C. et al. Retargeting of natural killer-cell cytolytic activity to ErbB2-expressing cancer cells results in efficient and selective tumor cell destruction. Blood 100, 1265–1273 (2002).
Smyth, M.J. et al. Nature's TRAIL–on a path to cancer immunotherapy. Immunity 18, 1–6 (2003).
Daniel, D. et al. Cooperation of the proapoptotic receptor agonist rhApo2L/TRAIL with the CD20 antibody rituximab against non-Hodgkin lymphoma xenografts. Blood 110, 4037–4046 (2007).
Taieb, J. et al. A novel dendritic cell subset involved in tumor immunosurveillance. Nat. Med. 12, 214–219 (2006).
Vosshenrich, C.A. et al. CD11cloB220+ interferon-producing killer dendritic cells are activated natural killer cells. J. Exp. Med. 204, 2569–2578 (2007).
Blasius, A.L., Barchet, W., Cella, M. & Colonna, M. Development and function of murine B220+CD11c+NK1.1+ cells identify them as a subset of NK cells. J. Exp. Med. 204, 2561–2568 (2007).
Caminschi, I. et al. Putative IKDCs are functionally and developmentally similar to natural killer cells, but not to dendritic cells. J. Exp. Med. 204, 2579–2590 (2007).
Chan, C.W. et al. Interferon-producing killer dendritic cells provide a link between innate and adaptive immunity. Nat. Med. 12, 207–213 (2006).
Bonmort, M. et al. Interferon-γ is produced by another player of innate immune responses: the interferon-producing killer dendritic cell (IKDC). Biochimie 89, 872–877 (2007).
Mailliard, R.B. et al. α-type-1 polarized dendritic cells: a novel immunization tool with optimized CTL-inducing activity. Cancer Res. 64, 5934–5937 (2004).
Osada, T., Clay, T., Hobeika, A., Lyerly, H.K. & Morse, M.A. NK cell activation by dendritic cell vaccine: a mechanism of action for clinical activity. Cancer Immunol. Immunother. 55, 1122–1131 (2006).
Andre, F. et al. Exosomes as potent cell-free peptide-based vaccine. I. Dendritic cell-derived exosomes transfer functional MHC class I/peptide complexes to dendritic cells. J. Immunol. 172, 2126–2136 (2004).
Chaput, N. et al. Exosomes as potent cell-free peptide-based vaccine. II. Exosomes in CpG adjuvants efficiently prime naive Tc1 lymphocytes leading to tumor rejection. J. Immunol. 172, 2137–2146 (2004).
Taieb, J. et al. Chemoimmunotherapy of tumors: cyclophosphamide synergizes with exosome based vaccines. J. Immunol. 176, 2722–2729 (2006).
Chaput, N. et al. Dendritic cell derived-exosomes: biology and clinical implementations. J. Leukoc. Biol. 80, 471–478 (2006).
Pilla, L. et al. A phase II trial of vaccination with autologous, tumor-derived heat-shock protein peptide complexes Gp96, in combination with GM-CSF and interferon-α in metastatic melanoma patients. Cancer Immunol. Immunother. 55, 958–968 (2006).
Sivori, S. et al. CpG and double-stranded RNA trigger human NK cells by Toll-like receptors: induction of cytokine release and cytotoxicity against tumors and dendritic cells. Proc. Natl. Acad. Sci. USA 101, 10116–10121 (2004).
Krieg, A.M., Efler, S.M., Wittpoth, M., Al Adhami, M.J. & Davis, H.L. Induction of systemic TH1-like innate immunity in normal volunteers following subcutaneous but not intravenous administration of CPG 7909, a synthetic B-class CpG oligodeoxynucleotide TLR9 agonist. J. Immunother. 27, 460–471 (2004).
Link, B.K. et al. Oligodeoxynucleotide CpG 7909 delivered as intravenous infusion demonstrates immunologic modulation in patients with previously treated non-Hodgkin lymphoma. J. Immunother. 29, 558–568 (2006).
Kanzler, H., Barrat, F.J., Hessel, E.M. & Coffman, R.L. Therapeutic targeting of innate immunity with Toll-like receptor agonists and antagonists. Nat. Med. 13, 552–559 (2007).
Whiteside, T.L. & Friberg, D. Natural killer cells and natural killer cell activity in chronic fatigue syndrome. Am. J. Med. 105, 27S–34S (1998).
Brandau, S. et al. NK cells are essential for effective BCG immunotherapy. Int. J. Cancer 92, 697–702 (2001).
Davies, F.E. et al. Thalidomide and immunomodulatory derivatives augment natural killer cell cytotoxicity in multiple myeloma. Blood 98, 210–216 (2001).
Brune, M. et al. Improved leukemia-free survival after postconsolidation immunotherapy with histamine dihydrochloride and interleukin-2 in acute myeloid leukemia: results of a randomized phase 3 trial. Blood 108, 88–96 (2006).
Ghiringhelli, F. et al. Metronomic cyclophosphamide regimen selectively depletes CD4+CD25+ regulatory T cells and restores T and NK effector functions in end stage cancer patients. Cancer Immunol. Immunother. 56, 641–648 (2007).
Ishikawa, A. et al. A phase I study of α-galactosylceramide (KRN7000)–pulsed dendritic cells in patients with advanced and recurrent non–small cell lung cancer. Clin. Cancer Res. 11, 1910–1917 (2005).
Motohashi, S. et al. A phase I study of in vitro expanded natural killer T cells in patients with advanced and recurrent non-small cell lung cancer. Clin. Cancer Res. 12, 6079–6086 (2006).
Mazodier, K. et al. Severe imbalance of IL-18/IL-18BP in patients with secondary hemophagocytic syndrome. Blood 106, 3483–3489 (2005).
Kim, M.H. et al. Secreted and membrane-associated matrix metalloproteinases of IL-2-activated NK cells and their inhibitors. J. Immunol. 164, 5883–5889 (2000).
Atkins, M.B. et al. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. J. Clin. Oncol. 17, 2105–2116 (1999).
Bukowski, R.M. Natural history and therapy of metastatic renal cell carcinoma: the role of interleukin-2. Cancer 80, 1198–1220 (1997).
Meropol, N.J. et al. Evaluation of natural killer cell expansion and activation in vivo with daily subcutaneous low-dose interleukin-2 plus periodic intermediate-dose pulsing. Cancer Immunol. Immunother. 46, 318–326 (1998).
Meseri, A. et al. Natural-killer cell activity and cytogenetic response in chronic myelogenous leukemia treated with alpha-interferon. Br. J. Haematol. 78, 585–586 (1991).
de Castro, F.A. et al. Immunological effects of interferon-alpha on chronic myelogenous leukemia. Leuk. Lymphoma 44, 2061–2067 (2003).
Gollob, J.A. et al. Phase I trial of twice-weekly intravenous interleukin 12 in patients with metastatic renal cell cancer or malignant melanoma: ability to maintain IFN-γ induction is associated with clinical response. Clin. Cancer Res. 6, 1678–1692 (2000).
Robertson, M.J. et al. Immunological effects of interleukin 12 administered by bolus intravenous injection to patients with cancer. Clin. Cancer Res. 5, 9–16 (1999).
Gollob, J.A. et al. Phase I trial of concurrent twice-weekly recombinant human interleukin-12 plus low-dose IL-2 in patients with melanoma or renal cell carcinoma. J. Clin. Oncol. 21, 2564–2573 (2003).
Robertson, M.J. et al. Clinical and biological effects of recombinant human interleukin-18 administered by intravenous infusion to patients with advanced cancer. Clin. Cancer Res. 12, 4265–4273 (2006).
Davis, I.D. et al. An open-label, two-arm, phase I trial of recombinant human interleukin-21 in patients with metastatic melanoma. Clin. Cancer Res. 13, 3630–3636 (2007).
Chen, W. et al. FLT3 ligand administration after hematopoietic cell transplantation increases circulating dendritic cell precursors that can be activated by CpG oligodeoxynucleotides to enhance T-cell and natural killer cell function. Biol. Blood Marrow Transplant. 11, 23–34 (2005).
Pilla, L. et al. Natural killer and NK-like T-cell activation in colorectal carcinoma patients treated with autologous tumor-derived heat shock protein 96. Cancer Res. 65, 3942–3949 (2005).
Mazzaferro, V. et al. Vaccination with autologous tumor-derived heat-shock protein gp96 after liver resection for metastatic colorectal cancer. Clin. Cancer Res. 9, 3235–3245 (2003).
McHutchison, J.G. et al. Phase 1B, randomized, double-blind, dose-escalation trial of CPG 10101 in patients with chronic hepatitis C virus. Hepatology 46, 1341–1349 (2007).
Escudier, B. et al. Vaccination of metastatic melanoma patients with autologous dendritic cell (DC) derived-exosomes: results of thefirst phase I clinical trial. J. Transl. Med. 3, 10 (2005).
Gluck, W.L. et al. Phase I studies of interleukin (IL)-2 and rituximab in B-cell non-Hodgkin's lymphoma: IL-2 mediated natural killer cell expansion correlations with clinical response. Clin. Cancer Res. 10, 2253–2264 (2004).
Berdeja, J.G. et al. Systemic interleukin-2 and adoptive transfer of lymphokine-activated killer cells improves antibody-dependent cellular cytotoxicity in patients with relapsed B-cell lymphoma treated with rituximab. Clin. Cancer Res. 13, 2392–2399 (2007).
Parihar, R. et al. A phase I study of interleukin 12 with trastuzumab in patients with human epidermal growth factor receptor-2-overexpressing malignancies: analysis of sustained interferon γ production in a subset of patients. Clin. Cancer Res. 10, 5027–5037 (2004).
Renner, C. et al. Initiation of humoral and cellular immune responses in patients with refractory Hodgkin's disease by treatment with an anti-CD16/CD30 bispecific antibody. Cancer Immunol. Immunother. 49, 173–180 (2000).
Hartmann, F. et al. Anti-CD16/CD30 bispecific antibody treatment for Hodgkin's disease: role of infusion schedule and costimulation with cytokines. Clin. Cancer Res. 7, 1873–1881 (2001).
Li, Z., Lim, W.K., Mahesh, S.P., Liu, B. & Nussenblatt, R.B. Cutting edge: in vivo blockade of human IL-2 receptor induces expansion of CD56bright regulatory NK cells in patients with active uveitis. J. Immunol. 174, 5187–5191 (2005).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Terme, M., Ullrich, E., Delahaye, N. et al. Natural killer cell–directed therapies: moving from unexpected results to successful strategies. Nat Immunol 9, 486–494 (2008). https://doi.org/10.1038/ni1580
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni1580
This article is cited by
-
Immune modulation in malignant pleural effusion: from microenvironment to therapeutic implications
Cancer Cell International (2024)
-
Intrathecal CAR-NK cells infusion for isolated CNS relapse after allogeneic stem cell transplantation: case report
Stem Cell Research & Therapy (2023)
-
Is neonatal uterine bleeding responsible for early-onset endometriosis?
Reproductive Biology and Endocrinology (2023)
-
Current status and perspective of CAR-T and CAR-NK cell therapy trials in Germany
Gene Therapy (2021)
-
Ex vivo expansion of autologous, donor-derived NK-, γδT-, and cytokine induced killer (CIK) cells post haploidentical hematopoietic stem cell transplantation results in increased antitumor activity
Bone Marrow Transplantation (2019)