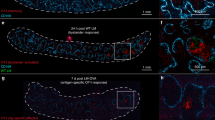
Abstract
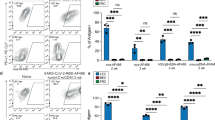
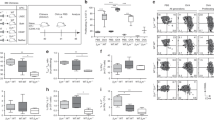
T lymphocytes lacking the lymph node–homing receptors L-selectin and CCR7 do not migrate to lymph nodes in the steady state. Instead, we found here that lymph nodes draining sites of mature dendritic cells or adjuvant inoculation recruited L-selectin-negative CCR7− effector and memory CD8+ T cells. This recruitment required CXCR3 expression on T cells and occurred through high endothelial venules in concert with lumenal expression of the CXCR3 ligand CXCL9. In reactive lymph nodes, recruited T cells established stable interactions with and killed antigen-bearing dendritic cells, limiting the ability of these dendritic cells to activate naive CD4+ and CD8+ T cells. The inducible recruitment of blood-borne effector and memory T cells to lymph nodes may represent a mechanism for terminating primary and limiting secondary immune responses.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Sallusto, F., Mackay, C.R. & Lanzavecchia, A. The role of chemokine receptors in primary, effector, and memory immune responses. Annu. Rev. Immunol. 18, 593–620 (2000).
Springer, T.A. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell 76, 301–314 (1994).
Butcher, E.C. & Picker, L.J. Lymphocyte homing and homeostasis. Science 272, 60–66 (1996).
Bajenoff, M. et al. Stromal cell networks regulate lymphocyte entry, migration, and territoriality in lymph nodes. Immunity 25, 989–1001 (2006).
Forster, R. et al. CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell 99, 23–33 (1999).
Lindquist, R.L. et al. Visualizing dendritic cell networks in vivo . Nat. Immunol. 5, 1243–1250 (2004).
Mempel, T.R., Henrickson, S.E. & Von Andrian, U.H. T-cell priming by dendritic cells in lymph nodes occurs in three distinct phases. Nature 427, 154–159 (2004).
von Andrian, U.H. & Mackay, C.R. T-cell function and migration. Two sides of the same coin. N. Engl. J. Med. 343, 1020–1034 (2000).
Austrup, F. et al. P- and E-selectin mediate recruitment of T-helper-1 but not T-helper-2 cells into inflammed tissues. Nature 385, 81–83 (1997).
Roman, E. et al. CD4 effector T cell subsets in the response to influenza: heterogeneity, migration, and function. J. Exp. Med. 196, 957–968 (2002).
Yoneyama, H. et al. Pivotal role of dendritic cell-derived CXCL10 in the retention of T helper cell 1 lymphocytes in secondary lymph nodes. J. Exp. Med. 195, 1257–1266 (2002).
Lawrence, C.W. & Braciale, T.J. Activation, differentiation, and migration of naive virus-specific CD8+ T cells during pulmonary influenza virus infection. J. Immunol. 173, 1209–1218 (2004).
Sallusto, F., Lenig, D., Forster, R., Lipp, M. & Lanzavecchia, A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 401, 708–712 (1999).
Reinhardt, R.L., Khoruts, A., Merica, R., Zell, T. & Jenkins, M.K. Visualizing the generation of memory CD4 T cells in the whole body. Nature 410, 101–105 (2001).
Masopust, D., Vezys, V., Marzo, A.L. & Lefrancois, L. Preferential localization of effector memory cells in nonlymphoid tissue. Science 291, 2413–2417 (2001).
Manjunath, N. et al. Effector differentiation is not prerequisite for generation of memory cytotoxic T lymphocytes. J. Clin. Invest. 108, 871–878 (2001).
Ferlazzo, G. et al. Human dendritic cells activate resting natural killer (NK) cells and are recognized via the NKp30 receptor by activated NK cells. J. Exp. Med. 195, 343–351 (2002).
Yang, J., Huck, S.P., McHugh, R.S., Hermans, I.F. & Ronchese, F. Perforin-dependent elimination of dendritic cells regulates the expansion of antigen-specific CD8+ T cells in vivo . Proc. Natl. Acad. Sci. USA 103, 147–152 (2006).
Chen, Q. et al. Fever-range thermal stress promotes lymphocyte trafficking across high endothelial venules via an interleukin 6 trans-signaling mechanism. Nat. Immunol. 7, 1299–1308 (2006).
Martin-Fontecha, A. et al. Induced recruitment of NK cells to lymph nodes provides IFN-γ for TH1 priming. Nat. Immunol. 5, 1260–1265 (2004).
Bajenoff, M. et al. Natural killer cell behavior in lymph nodes revealed by static and real-time imaging. J. Exp. Med. 203, 619–631 (2006).
Yoneyama, H. et al. Evidence for recruitment of plasmacytoid dendritic cell precursors to inflamed lymph nodes through high endothelial venules. Int. Immunol. 16, 915–928 (2004).
Hadida, F. et al. Carboxyl-terminal and central regions of human immunodeficiency virus-1 NEF recognized by cytotoxic T lymphocytes from lymphoid organs. An in vitro limiting dilution analysis. J. Clin. Invest. 89, 53–60 (1992).
Hosmalin, A. et al. HIV-specific effector cytotoxic T lymphocytes and HIV-producing cells colocalize in white pulps and germinal centers from infected patients. Blood 97, 2695–2701 (2001).
Kyburz, D., Speiser, D.E., Aebischer, T., Hengartner, H. & Zinkernagel, R.M. Virus-specific cytotoxic T cell-mediated lysis of lymphocytes in vitro and in vivo . J. Immunol. 150, 5051–5058 (1993).
Odermatt, B., Eppler, M., Leist, T.P., Hengartner, H. & Zinkernagel, R.M. Virus-triggered acquired immunodeficiency by cytotoxic T-cell-dependent destruction of antigen-presenting cells and lymph follicle structure. Proc. Natl. Acad. Sci. USA 88, 8252–8256 (1991).
Borrow, P., Evans, C.F. & Oldstone, M.B. Virus-induced immunosuppression: immune system-mediated destruction of virus-infected dendritic cells results in generalized immune suppression. J. Virol. 69, 1059–1070 (1995).
Galkina, E. et al. Preferential migration of effector CD8+ T cells into the interstitium of the normal lung. J. Clin. Invest. 115, 3473–3483 (2005).
Hawiger, D. et al. Dendritic cells induce peripheral T cell unresponsiveness under steady state conditions in vivo . J. Exp. Med. 194, 769–779 (2001).
Gett, A.V., Sallusto, F., Lanzavecchia, A. & Geginat, J. T cell fitness determined by signal strength. Nat. Immunol. 4, 355–360 (2003).
Mackay, C.R., Marston, W.L. & Dudler, L. Naive and memory T cells show distinct pathways of lymphocyte recirculation. J. Exp. Med. 171, 801–817 (1990).
Debes, G.F. et al. Chemokine receptor CCR7 required for T lymphocyte exit from peripheral tissues. Nat. Immunol. 6, 889–894 (2005).
Bromley, S.K., Thomas, S.Y. & Luster, A.D. Chemokine receptor CCR7 guides T cell exit from peripheral tissues and entry into afferent lymphatics. Nat. Immunol. 6, 895–901 (2005).
Martin-Fontecha, A. et al. Regulation of dendritic cell migration to the draining lymph node: impact on T lymphocyte traffic and priming. J. Exp. Med. 198, 615–621 (2003).
Stein, J.V. et al. The CC chemokine thymus-derived chemotactic agent 4 (TCA-4, secondary lymphoid tissue chemokine, 6Ckine, exodus-2) triggers lymphocyte function-associated antigen 1-mediated arrest of rolling T lymphocytes in peripheral lymph node high endothelial venules. J. Exp. Med. 191, 61–76 (2000).
Palframan, R.T. et al. Inflammatory chemokine transport and presentation in HEV: a remote control mechanism for monocyte recruitment to lymph nodes in inflamed tissues. J. Exp. Med. 194, 1361–1373 (2001).
von Andrian, U.H., Hasslen, S.R., Nelson, R.D., Erlandsen, S.L. & Butcher, E.C. A central role for microvillous receptor presentation in leukocyte adhesion under flow. Cell 82, 989–999 (1995).
Stein, J.V. et al. L-selectin-mediated leukocyte adhesion in vivo: microvillous distribution determines tethering efficiency, but not rolling velocity. J. Exp. Med. 189, 37–50 (1999).
Ronchese, F. & Hermans, I.F. Killing of dendritic cells: a life cut short or a purposeful death? J. Exp. Med. 194, F23–F26 (2001).
Wilson, J.L. et al. Targeting of human dendritic cells by autologous NK cells. J. Immunol. 163, 6365–6370 (1999).
Medema, J.P. et al. Expression of the serpin serine protease inhibitor 6 protects dendritic cells from cytotoxic T lymphocyte-induced apoptosis: differential modulation by T helper type 1 and type 2 cells. J. Exp. Med. 194, 657–667 (2001).
Hermans, I.F., Ritchie, D.S., Yang, J., Roberts, J.M. & Ronchese, F. CD8+ T cell-dependent elimination of dendritic cells in vivo limits the induction of antitumor immunity. J. Immunol. 164, 3095–3101 (2000).
Matloubian, M. et al. A role for perforin in downregulating T-cell responses during chronic viral infection. J. Virol. 73, 2527–2536 (1999).
Badovinac, V.P. & Harty, J.T. Adaptive immunity and enhanced CD8+ T cell response to Listeria monocytogenes in the absence of perforin and IFN-γ. J. Immunol. 164, 6444–6452 (2000).
Iezzi, G., Karjalainen, K. & Lanzavecchia, A. The duration of antigenic stimulation determines the fate of naive and effector T cells. Immunity 8, 89–95 (1998).
Klenerman, P. & Zinkernagel, R.M. Original antigenic sin impairs cytotoxic T lymphocyte responses to viruses bearing variant epitopes. Nature 394, 482–485 (1998).
Traunecker, A., Oliveri, F. & Karjalainen, K. Myeloma based expression system for production of large mammalian proteins. Trends Biotechnol. 9, 109–113 (1991).
Clarke, S.R. et al. Characterization of the ovalbumin-specific TCR transgenic line OT-I: MHC elements for positive and negative selection. Immunol. Cell Biol. 78, 110–117 (2000).
Barnden, M.J., Allison, J., Heath, W.R. & Carbone, F.R. Defective TCR expression in transgenic mice constructed using cDNA-based α- and β-chain genes under the control of heterologous regulatory elements. Immunol. Cell Biol. 76, 34–40 (1998).
Hancock, W.W. et al. Requirement of the chemokine receptor CXCR3 for acute allograft rejection. J. Exp. Med. 192, 1515–1520 (2000).
Pope, C. et al. Organ-specific regulation of the CD8 T cell response to Listeria monocytogenes infection. J. Immunol. 166, 3402–3409 (2001).
von Andrian, U.H. Intravital microscopy of the peripheral lymph node microcirculation in mice. Microcirculation 3, 287–300 (1996).
Nombela-Arrieta, C. et al. Differential requirements for DOCK2 and phosphoinositide-3-kinase γ during T and B lymphocyte homing. Immunity 21, 429–441 (2004).
Castellino, F. et al. Chemokines enhance immunity by guiding naive CD8+ T cells to sites of CD4+ T cell-dendritic cell interaction. Nature 440, 890–895 (2006).
Acknowledgements
We thank L. Lefrancois (University of Connecticut) for the Listeria monocytogenes strain expressing OVA; P. Dellabona (DIBIT San Raffaele Research Institute), C. Gerard (Harvard Medical School), J. Kirberg (Max Planck Institute) and M. Lipp (Max Delbruck Center) for transgenic and knockout mice; M. Manz and M. Uguccioni for critical reading and comments; A. Almeida for discussions; and D. Jarrossay, E. Mira-Catò, L. Perlini and M. Convert for technical help. Supported by the Swiss National Science Foundation (31-101962 and 31-109832), the European Commission FP6 'Network of Excellence' initiative (N. LSHG-CT-2003-502935 MAIN, LSHB-CT-2004-512074 DC-THERA and LSHG-CT-2005-005203 MUGEN), the intramural program of the National Institute of Allergy and Infectious Diseases of the National Institutes of Health, the Cancer Research Institute (A.H.) and the Helmut Horten Foundation (for the Institute for Research in Biomedicine).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
Priming of naive CD4+ T cells is partially recovered in mice carrying effector or memory CD8+ T cells when MHC class I and class II peptides are provided on different DCs. (PDF 50 kb)
Supplementary Video 1
Dynamic intravital 2-photon imaging movies of prolonged T cell-DC interaction followed by DC cell body fragmentation. SIINFEKL pulsed-DCs (green) and control peptide pulsed-DCs (purple) were activated with LPS and co-injected in the footpad of recipient mice 24 hours prior to intravenous injection of in vitro-generated L-selectin− effector OT-I cells (red). Intravital 2-photon microscopy imaging was performed on the draining popliteal lymph node of anesthetized recipient animals in the interfollicular region 60-160 μm below the capsule starting at 3 hours after adoptive transfer of T cells. An example of prolonged T cell interaction with SIINFEKL pulsed-DCs is shown (highlighted by dotted circle). These prolonged interactions are followed by fragmentation of the DC cell body over the subsequent several minutes. Control peptide pulsed-DCs (purple) are either ignored by or exhibit transient interaction with T cells, and did not exhibit any fragmentation during the same observation period. Clock (h:min:sec) denotes the time after i.v. OT-I cell transfer. Scale bar as indicated. Playback speed: 450X. Total time: 3 hrs 18 mins. (AVI 7938 kb)
Supplementary Video 2
Dynamic intravital 2-photon imaging movies of prolonged T cell-DC interaction followed by DC cell body fragmentation. SIINFEKL pulsed-DCs (green) and control peptide pulsed-DCs (purple) were activated with LPS and co-injected in the footpad of recipient mice 24 hours prior to intravenous injection of in vitro-generated L-selectin− effector OT-I cells (red). Intravital 2-photon microscopy imaging was performed on the draining popliteal lymph node of anesthetized recipient animals in the interfollicular region 60-160 μm below the capsule starting at 3 hours after adoptive transfer of T cells. Shown is an example of prolonged T cell interaction with SIINFEKL pulsed-DCs followed by fragmentation of the DC cell body. Clock (h:min:sec) denotes the time after i.v. OT-I cell transfer. Scale bar as indicated. Playback speed: 450X. Total time: 1 hr 8 mins 45 secs (Movie 2). (AVI 1206 kb)
Rights and permissions
About this article
Cite this article
Guarda, G., Hons, M., Soriano, S. et al. L-selectin-negative CCR7− effector and memory CD8+ T cells enter reactive lymph nodes and kill dendritic cells. Nat Immunol 8, 743–752 (2007). https://doi.org/10.1038/ni1469
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni1469
This article is cited by
-
Localization, tissue biology and T cell state — implications for cancer immunotherapy
Nature Reviews Immunology (2023)
-
High endothelial venules (HEVs) in immunity, inflammation and cancer
Angiogenesis (2021)
-
T-lymphocyte homing: an underappreciated yet critical hurdle for successful cancer immunotherapy
Laboratory Investigation (2017)
-
Mechanisms of T cell organotropism
Cellular and Molecular Life Sciences (2016)
-
SOCS4 is dispensable for an efficient recall response to influenza despite being required for primary immunity
Immunology & Cell Biology (2015)