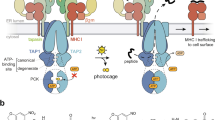
Abstract
Heterogeneous intracellular pathways and biochemical mechanisms are responsible for generating the glycoprotein complexes of peptide and major histocompatibility complex that are displayed on the surfaces of antigen-presenting cells for recognition by T lymphocytes. These pathways have a profound influence on the specificity of adaptive immunity and tolerance, as well as the context and consequences of antigen recognition by T cells in the thymus and periphery. The field of antigen processing and presentation has continued to advance since the publication of a focus issue on the topic in Nature Immunology in July 2004. Progress has been made on many fronts, including advances in understanding how proteases, accessory molecules and intracellular pathways influence peptide loading and antigen presentation in various cell types.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Kloetzel, P.M. Generation of major histocompatibility complex class I antigens: functional interplay between proteasomes and TPPII. Nat. Immunol. 5, 661–669 (2004).
Rock, K.L., York, I.A. & Goldberg, A.L. Post-proteasomal antigen processing for major histocompatibility complex class I presentation. Nat. Immunol. 5, 670–677 (2004).
Watts, C. The exogenous pathway for antigen presentation on major histocompatibility complex class II and CD1 molecules. Nat. Immunol. 5, 685–692 (2004).
Ackerman, A.L. & Cresswell, P. Cellular mechanisms governing cross-presentation of exogenous antigens. Nat. Immunol. 5, 678–684 (2004).
Stern, L.J. & Wiley, D.C. Antigenic peptide binding by class I and class II histocompatibility proteins. Structure 2, 245–251 (1994).
Glithero, A. et al. The crystal structure of H-2Db complexed with a partial peptide epitope suggests a major histocompatibility complex class I assembly intermediate. J. Biol. Chem. 281, 12699–12704 (2006).
Carven, G.J. et al. Monoclonal antibodies specific for the empty conformation of HLA-DR1 reveal aspects of the conformational change associated with peptide binding. J. Biol. Chem. 279, 16561–16570 (2004).
Cresswell, P., Ackerman, A.L., Giodini, A., Peaper, D.R. & Wearsch, P.A. Mechanisms of MHC class I-restricted antigen processing and cross-presentation. Immunol. Rev. 207, 145–157 (2005).
Jensen, P.E., Weber, D.A., Thayer, W.P., Westerman, L.E. & Dao, C.T. Peptide exchange in MHC molecules. Immunol. Rev. 172, 229–238 (1999).
Cresswell, P. Invariant chain structure and MHC class II function. Cell 84, 505–507 (1996).
Carven, G.J. & Stern, L.J. Probing the ligand-induced conformational change in HLA-DR1 by selective chemical modification and mass spectrometric mapping. Biochemistry 44, 13625–13637 (2005).
Weber, D.A., Evavold, B.D. & Jensen, P.E. Enhanced dissociation of HLA-DR-bound peptides in the presence of HLA-DM. Science 274, 618–620 (1996).
Pashine, A. et al. Interaction of HLA-DR with an acidic face of HLA-DM disrupts sequence-dependent interactions with peptides. Immunity 19, 183–192 (2003).
Belmares, M.P., Busch, R., Wucherpfennig, K.W., McConnell, H.M. & Mellins, E.D. Structural factors contributing to DM susceptibility of MHC class II-peptide complexes. J. Immunol. 169, 5109–5117 (2002).
Chou, C.L. & Sadegh-Nasseri, S. HLA-DM recognizes the flexible conformation of major histocompatibility complex class II. J. Exp. Med. 192, 1697–1706 (2000).
Lazarski, C.A. et al. The kinetic stability of MHC class II:peptide complexes is a key parameter that dictates immunodominance. Immunity 23, 29–40 (2005).
Lazarski, C.A., Chaves, F.A. & Sant, A.J. The impact of DM on MHC class II-restricted antigen presentation can be altered by manipulation of MHC-peptide kinetic stability. J. Exp. Med. 203, 1319–1328 (2006).
McFarland, B.J., Katz, J.F., Sant, A.J. & Beeson, C. Energetics and cooperativity of the hydrogen bonding and anchor interactions that bind peptides to MHC class II protein. J. Mol. Biol. 350, 170–183 (2005).
Narayan, K. et al. HLA-DM targets the hydrogen bond between the histidine at position β81 and peptide to dissociate HLA-DR-peptide complexes. Nat. Immunol. 8, 92–100 (2007).
Lovitch, S.B., Pu, Z. & Unanue, E.R. Amino-terminal flanking residues determine the conformation of a peptide-class II MHC complex. J. Immunol. 176, 2958–2968 (2006).
Lovitch, S.B., Esparza, T.J., Schweitzer, G., Herzog, J. & Unanue, E.R. Activation of type B T cells after protein immunization reveals novel pathways of in vivo presentation of peptides. J. Immunol. 178, 122–133 (2007).
De Wall, S.L. et al. Noble metals strip peptides from class II MHC proteins. Nat. Chem. Biol. 2, 197–201 (2006).
Hornell, T.M. et al. Human dendritic cell expression of HLA-DO is subset specific and regulated by maturation. J. Immunol. 176, 3536–3547 (2006).
Chen, X., Reed-Loisel, L.M., Karlsson, L. & Jensen, P.E. H2-O expression in primary dendritic cells. J. Immunol. 176, 3548–3556 (2006).
Fallas, J.L., Yi, W., Draghi, N.A., O'Rourke, H.M. & Denzin, L.K. Expression patterns of H2-O in mouse B cells and dendritic cells correlate with cell function. J. Immunol. 178, 1488–1497 (2007).
Chen, X. et al. Regulated expression of human histocompatibility leukocyte antigen (HLA)-DO during antigen-dependent and antigen-independent phases of B cell development. J. Exp. Med. 195, 1053–1062 (2002).
Glazier, K.S. et al. Germinal center B cells regulate their capability to present antigen by modulation of HLA-DO. J. Exp. Med. 195, 1063–1069 (2002).
Maric, M. et al. Defective antigen processing in GILT-free mice. Science 294, 1361–1365 (2001).
Delamarre, L., Pack, M., Chang, H., Mellman, I. & Trombetta, E.S. Differential lysosomal proteolysis in antigen-presenting cells determines antigen fate. Science 307, 1630–1634 (2005).
Delamarre, L., Couture, R., Mellman, I. & Trombetta, E.S. Enhancing immunogenicity by limiting susceptibility to lysosomal proteolysis. J. Exp. Med. 203, 2049–2055 (2006).
Sercarz, E.E. & Maverakis, E. Mhc-guided processing: binding of large antigen fragments. Nat. Rev. Immunol. 3, 621–629 (2003).
Mimura, Y. et al. Folding of an MHC class II-restricted tumor antigen controls its antigenicity via MHC-guided processing. Proc. Natl. Acad. Sci. USA 104, 5983–5988 (2007).
Davidson, H.W. & Watts, C. Epitope-directed processing of specific antigen by B lymphocytes. J. Cell Biol. 109, 85–92 (1989).
Simitsek, P.D., Campbell, D.G., Lanzavecchia, A., Fairweather, N. & Watts, C. Modulation of antigen processing by bound antibodies can boost or suppress class II major histocompatibility complex presentation of different T cell determinants. J. Exp. Med. 181, 1957–1963 (1995).
Moss, C.X., Tree, T.I. & Watts, C. Reconstruction of a pathway of antigen processing and class II MHC peptide capture. EMBO J. 26, 2137–2147 (2007).
Trombetta, E.S., Ebersold, M., Garrett, W., Pypaert, M. & Mellman, I. Activation of lysosomal function during dendritic cell maturation. Science 299, 1400–1403 (2003).
West, M.A. et al. Enhanced dendritic cell antigen capture via Toll-like receptor-induced actin remodeling. Science 305, 1153–1157 (2004).
Santambrogio, L. et al. Involvement of caspase-cleaved and intact adaptor protein 1 complex in endosomal remodeling in maturing dendritic cells. Nat. Immunol. 6, 1020–1028 (2005).
Shin, J.S. et al. Surface expression of MHC class II in dendritic cells is controlled by regulated ubiquitination. Nature 444, 115–118 (2006).
van Niel, G. et al. Dendritic cells regulate exposure of MHC class II at their plasma membrane by oligoubiquitination. Immunity 25, 885–894 (2006).
Ohmura-Hoshino, M. et al. Inhibition of MHC class II expression and immune responses by c-MIR. J. Immunol. 177, 341–354 (2006).
Matsuki, Y. et al. Novel regulation of MHC class II function in B cells. EMBO J. 26, 846–854 (2007).
Blander, J.M. & Medzhitov, R. Toll-dependent selection of microbial antigens for presentation by dendritic cells. Nature 440, 808–812 (2006).
Blander, J.M. & Medzhitov, R. Regulation of phagosome maturation by signals from Toll-like receptors. Science 304, 1014–1018 (2004).
Elliott, T. & Williams, A. The optimization of peptide cargo bound to MHC class I molecules by the peptide-loading complex. Immunol. Rev. 207, 89–99 (2005).
Sijts, A.J. & Pamer, E.G. Enhanced intracellular dissociation of major histocompatibility complex class I-associated peptides: a mechanism for optimizing the spectrum of cell surface-presented cytotoxic T lymphocyte epitopes. J. Exp. Med. 185, 1403–1411 (1997).
Lewis, J.W. & Elliott, T. Evidence for successive peptide binding and quality control stages during MHC class I assembly. Curr. Biol. 8, 717–720 (1998).
Howarth, M., Williams, A., Tolstrup, A.B. & Elliott, T. Tapasin enhances MHC class I peptide presentation according to peptide half-life. Proc. Natl. Acad. Sci. USA 101, 11737–11742 (2004).
Paulsson, K.M., Jevon, M., Wang, J.W., Li, S. & Wang, P. The double lysine motif of tapasin is a retrieval signal for retention of unstable MHC class I molecules in the endoplasmic reticulum. J. Immunol. 176, 7482–7488 (2006).
Dick, T.P., Bangia, N., Peaper, D.R. & Cresswell, P. Disulfide bond isomerization and the assembly of MHC class I-peptide complexes. Immunity 16, 87–98 (2002).
Peaper, D.R., Wearsch, P.A. & Cresswell, P. Tapasin and ERp57 form a stable disulfide-linked dimer within the MHC class I peptide-loading complex. EMBO J. 24, 3613–3623 (2005).
Garbi, N., Tanaka, S., Momburg, F. & Hammerling, G.J. Impaired assembly of the major histocompatibility complex class I peptide-loading complex in mice deficient in the oxidoreductase ERp57. Nat. Immunol. 7, 93–102 (2006).
Kienast, A., Preuss, M., Winkler, M. & Dick, T.P. Redox regulation of peptide receptivity of major histocompatibility complex class I molecules by ERp57 and tapasin. Nat. Immunol. 8, 864–872 (2007).
Chen, M. & Bouvier, M. Analysis of interactions in a tapasin-class I complex provides a mechanism for peptide selection. EMBO J. 26, 1681–1690 (2007).
Wearsch, P.A. & Cresswell, P. Selective loading of high-affinity peptides onto major histocompatibility complex class I molecules by the tapasin-ERp57 heterodimer. Nat. Immunol. 8, 873–881 (2007).
Park, B. et al. Redox regulation facilitates optimal peptide selection by MHC class I during antigen processing. Cell 127, 369–382 (2006).
York, I.A., Bhutani, N., Zendzian, S., Goldberg, A.L. & Rock, K.L. Tripeptidyl peptidase II is the major peptidase needed to trim long antigenic precursors, but is not required for most MHC class I antigen presentation. J. Immunol. 177, 1434–1443 (2006).
Schubert, U. et al. Rapid degradation of a large fraction of newly synthesized proteins by proteasomes. Nature 404, 770–774 (2000).
Yewdell, J.W. & Nicchitta, C.V. The DRiP hypothesis decennial: support, controversy, refinement and extension. Trends Immunol. 27, 368–373 (2006).
Yewdell, J.W. The seven dirty little secrets of major histocompatibility complex class I antigen processing. Immunol. Rev. 207, 8–18 (2005).
Vabulas, R.M. & Hartl, F.U. Protein synthesis upon acute nutrient restriction relies on proteasome function. Science 310, 1960–1963 (2005).
Zook, M.B., Howard, M.T., Sinnathamby, G., Atkins, J.F. & Eisenlohr, L.C. Epitopes derived by incidental translational frameshifting give rise to a protective CTL response. J. Immunol. 176, 6928–6934 (2006).
Hanada, K., Yewdell, J.W. & Yang, J.C. Immune recognition of a human renal cancer antigen through post-translational protein splicing. Nature 427, 252–256 (2004).
Vigneron, N. et al. An antigenic peptide produced by peptide splicing in the proteasome. Science 304, 587–590 (2004).
Warren, E.H. et al. An antigen produced by splicing of noncontiguous peptides in the reverse order. Science 313, 1444–1447 (2006).
Shastri, N., Schwab, S. & Serwold, T. Producing nature's gene-chips: the generation of peptides for display by MHC class I molecules. Annu. Rev. Immunol. 20, 463–493 (2002).
Reits, E. et al. A major role for TPPII in trimming proteasomal degradation products for MHC class I antigen presentation. Immunity 20, 495–506 (2004).
Guil, S. et al. Need for tripeptidyl-peptidase II in major histocompatibility complex class I viral antigen processing when proteasomes are detrimental. J. Biol. Chem. 281, 39925–39934 (2006).
Serwold, T., Gonzalez, F., Kim, J., Jacob, R. & Shastri, N. ERAAP customizes peptides for MHC class I molecules in the endoplasmic reticulum. Nature 419, 480–483 (2002).
Saric, T. et al. An IFN-gamma-induced aminopeptidase in the ER, ERAP1, trims precursors to MHC class I-presented peptides. Nat. Immunol. 3, 1169–1176 (2002).
York, I.A. et al. The ER aminopeptidase ERAP1 enhances or limits antigen presentation by trimming epitopes to 8–9 residues. Nat. Immunol. 3, 1177–1184 (2002).
Chang, S.C., Momburg, F., Bhutani, N. & Goldberg, A.L. The ER aminopeptidase, ERAP1, trims precursors to lengths of MHC class I peptides by a “molecular ruler” mechanism. Proc. Natl. Acad. Sci. USA 102, 17107–17112 (2005).
Kanaseki, T., Blanchard, N., Hammer, G.E., Gonzalez, F. & Shastri, N. ERAAP synergizes with MHC class I molecules to make the final cut in the antigenic peptide precursors in the endoplasmic reticulum. Immunity 25, 795–806 (2006).
Reits, E. et al. Peptide diffusion, protection, and degradation in nuclear and cytoplasmic compartments before antigen presentation by MHC class I. Immunity 18, 97–108 (2003).
Spee, P. & Neefjes, J. TAP-translocated peptides specifically bind proteins in the endoplasmic reticulum, including gp96, protein disulfide isomerase and calreticulin. Eur. J. Immunol. 27, 2441–2449 (1997).
Lammert, E., Stevanovic, S., Brunner, J., Rammensee, H.G. & Schild, H. Protein disulfide isomerase is the dominant acceptor for peptides translocated into the endoplasmic reticulum. Eur. J. Immunol. 27, 1685–1690 (1997).
Saveanu, L. et al. Concerted peptide trimming by human ERAP1 and ERAP2 aminopeptidase complexes in the endoplasmic reticulum. Nat. Immunol. 6, 689–697 (2005).
Hammer, G.E., Gonzalez, F., Champsaur, M., Cado, D. & Shastri, N. The aminopeptidase ERAAP shapes the peptide repertoire displayed by major histocompatibility complex class I molecules. Nat. Immunol. 7, 103–112 (2006).
Yan, J. et al. In vivo role of ER-associated peptidase activity in tailoring peptides for presentation by MHC class Ia and class Ib molecules. J. Exp. Med. 203, 647–659 (2006).
York, I.A., Brehm, M.A., Zendzian, S., Towne, C.F. & Rock, K.L. Endoplasmic reticulum aminopeptidase 1 (ERAP1) trims MHC class I-presented peptides in vivo and plays an important role in immunodominance. Proc. Natl. Acad. Sci. USA 103, 9202–9207 (2006).
Hammer, G.E., Gonzalez, F., James, E., Nolla, H. & Shastri, N. In the absence of aminopeptidase ERAAP, MHC class I molecules present many unstable and highly immunogenic peptides. Nat. Immunol. 8, 101–108 (2007).
Murata, S. et al. Regulation of CD8+ T cell development by thymus-specific proteasomes. Science 316, 1349–1353 (2007).
Nakagawa, T. et al. Cathepsin L: critical role in Ii degradation and CD4 T cell selection in the thymus. Science 280, 450–453 (1998).
Groothuis, T.A. & Neefjes, J. The many roads to cross-presentation. J. Exp. Med. 202, 1313–1318 (2005).
Bevan, M.J. Cross-priming. Nat. Immunol. 7, 363–365 (2006).
Shen, L. & Rock, K.L. Priming of T cells by exogenous antigen cross-presented on MHC class I molecules. Curr. Opin. Immunol. 18, 85–91 (2006).
den Haan, J.M., Lehar, S.M. & Bevan, M.J. CD8+ but not CD8− dendritic cells cross-prime cytotoxic T cells in vivo. J. Exp. Med. 192, 1685–1696 (2000).
Iyoda, T. et al. The CD8+ dendritic cell subset selectively endocytoses dying cells in culture and in vivo. J. Exp. Med. 195, 1289–1302 (2002).
Dudziak, D. et al. Differential antigen processing by dendritic cell subsets in vivo. Science 315, 107–111 (2007).
Shen, L., Sigal, L.J., Boes, M. & Rock, K.L. Important role of cathepsin S in generating peptides for TAP-independent MHC class I crosspresentation in vivo. Immunity 21, 155–165 (2004).
Norbury, C.C. et al. CD8+ T cell cross-priming via transfer of proteasome substrates. Science 304, 1318–1321 (2004).
Wolkers, M.C., Brouwenstijn, N., Bakker, A.H., Toebes, M. & Schumacher, T.N. Antigen bias in T cell cross-priming. Science 304, 1314–1317 (2004).
Shen, L. & Rock, K.L. Cellular protein is the source of cross-priming antigen in vivo. Proc. Natl. Acad. Sci. USA 101, 3035–3040 (2004).
Srivastava, P. Interaction of heat shock proteins with peptides and antigen presenting cells: chaperoning of the innate and adaptive immune responses. Annu. Rev. Immunol. 20, 395–425 (2002).
Accapezzato, D. et al. Chloroquine enhances human CD8+ T cell responses against soluble antigens in vivo. J. Exp. Med. 202, 817–828 (2005).
Savina, A. et al. NOX2 controls phagosomal pH to regulate antigen processing during crosspresentation by dendritic cells. Cell 126, 205–218 (2006).
Ackerman, A.L., Giodini, A. & Cresswell, P. A role for the endoplasmic reticulum protein retrotranslocation machinery during crosspresentation by dendritic cells. Immunity 25, 607–617 (2006).
Wiertz, E.J. et al. Sec61-mediated transfer of a membrane protein from the endoplasmic reticulum to the proteasome for destruction. Nature 384, 432–438 (1996).
Ackerman, A.L., Kyritsis, C. & Tamp, È.R. & Cresswell, P. Access of soluble antigens to the endoplasmic reticulum can explain cross-presentation by dendritic cells. Nat. Immunol. 6, 107–113 (2005).
Burgdorf, S., Kautz, A., Bohnert, V., Knolle, P.A. & Kurts, C. Distinct pathways of antigen uptake and intracellular routing in CD4 and CD8 T cell activation. Science 316, 612–616 (2007).
Gagnon, E. et al. Endoplasmic reticulum-mediated phagocytosis is a mechanism of entry into macrophages. Cell 110, 119–131 (2002).
Guermonprez, P. et al. ER-phagosome fusion defines an MHC class I cross-presentation compartment in dendritic cells. Nature 425, 397–402 (2003).
Houde, M. et al. Phagosomes are competent organelles for antigen cross-presentation. Nature 425, 402–406 (2003).
Hatsuzawa, K. et al. Involvement of syntaxin 18, an endoplasmic reticulum (ER)-localized SNARE protein, in ER-mediated phagocytosis. Mol. Biol. Cell 17, 3964–3977 (2006).
Touret, N. et al. Quantitative and dynamic assessment of the contribution of the ER to phagosome formation. Cell 123, 157–170 (2005).
Neijssen, J. et al. Cross-presentation by intercellular peptide transfer through gap junctions. Nature 434, 83–88 (2005).
Zhou, D. et al. Lamp-2a facilitates MHC class II presentation of cytoplasmic antigens. Immunity 22, 571–581 (2005).
Dengjel, J. et al. Autophagy promotes MHC class II presentation of peptides from intracellular source proteins. Proc. Natl. Acad. Sci. USA 102, 7922–7927 (2005).
Schmid, D., Pypaert, M. & Munz, C. Antigen-loading compartments for major histocompatibility complex class II molecules continuously receive input from autophagosomes. Immunity 26, 79–92 (2007).
Tewari, M.K., Sinnathamby, G., Rajagopal, D. & Eisenlohr, L.C. A cytosolic pathway for MHC class II-restricted antigen processing that is proteasome and TAP dependent. Nat. Immunol. 6, 287–294 (2005).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The author declares no competing financial interests.
Rights and permissions
About this article
Cite this article
Jensen, P. Recent advances in antigen processing and presentation. Nat Immunol 8, 1041–1048 (2007). https://doi.org/10.1038/ni1516
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni1516
This article is cited by
-
Extensive MHC class IIβ diversity across multiple loci in the small-spotted catshark (Scyliorhinus canicula)
Scientific Reports (2023)
-
Pan-genome and reverse vaccinology approaches to design multi-epitope vaccine against Epstein-Barr virus associated with colorectal cancer
Immunologic Research (2023)
-
Immunogenetic variation shapes the gut microbiome in a natural vertebrate population
Microbiome (2022)
-
A transformer-based model to predict peptide–HLA class I binding and optimize mutated peptides for vaccine design
Nature Machine Intelligence (2022)
-
A guide to antigen processing and presentation
Nature Reviews Immunology (2022)