Abstract
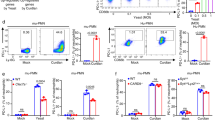
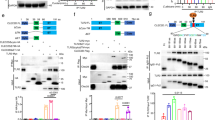
Dectin-1 is a C-type lectin involved in the recognition of β-glucans found in the cell walls of fungi. We generated dectin-1-deficient mice to determine the importance of dectin-1 in the defense against pathogenic fungi. In vitro, β-glucan-induced cytokine production from wild-type dendritic cells and macrophages was abolished in cells homozygous for dectin-1 deficiency ('dectin-1-knockout' cells). In vivo, dectin-1-knockout mice were more susceptible than wild-type mice to pneumocystis infection, even though their cytokine production was normal. However, pneumocystis-infected dectin-1-knockout macrophages did show defective production of reactive oxygen species. In contrast to those results, wild-type and dectin-1-knockout mice were equally susceptible to candida infection. Thus, dectin-1 is required for immune responses to some fungal infections, as protective immunity to pneumocystis, but not to candida, required dectin-1 for the production of antifungal reactive oxygen species.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Figdor, C.G., van Kooyk, Y. & Adema, G.J. C-type lectin receptors on dendritic cells and Langerhans cells. Nat. Rev. Immunol. 2, 77–84 (2002).
Ariizumi, K. et al. Identification of a novel, dendritic cell-associated molecule, dectin-1, by subtractive cDNA cloning. J. Biol. Chem. 275, 20157–20167 (2000).
Adachi, Y. et al. Characterization of β-glucan recognition site on C-type lectin, dectin 1. Infect. Immun. 72, 4159–4171 (2004).
Taylor, P.R. et al. The β-glucan receptor, dectin-1, is predominantly expressed on the surface of cells of the monocyte/macrophage and neutrophil lineages. J. Immunol. 169, 3876–3882 (2002).
Brown, G.D. & Gordon, S. Fungal β-glucans and mammalian immunity. Immunity 19, 311–315 (2003).
Fitzpatrick, F.W., Haynes, L.J., Silver, N.J. & Dicarlo, F.J. Effect of glucan derivatives upon phagocytosis by mice. J. Reticuloendothel. Soc. 15, 423–428 (1964).
Ross, G.D., Vetvicka, V., Yan, J., Xia, Y. & Vetvickova, J. Therapeutic intervention with complement and β-glucan in cancer. Immunopharmacology 42, 61–74 (1999).
Steele, C. et al. Alveolar macrophage-mediated killing of Pneumocystis carinii f. sp. muris involves molecular recognition by the dectin-1 β-glucan receptor. J. Exp. Med. 198, 1677–1688 (2003).
Gantner, B.N., Simmons, R.M. & Underhill, D.M. Dectin-1 mediates macrophage recognition of Candida albicans yeast but not filaments. EMBO J. 24, 1277–1286 (2005).
Romani, L. Immunity to fungal infections. Nat. Rev. Immunol. 4, 1–23 (2004).
Odds, F.C. Candia and Candidosis 2nd edn. (ed. Odds, F.C.) 252–278 (Baillere-Tindall, London, 1988).
Villamon, E., Roig, P., Gil, M.L. & Gozalbo, D. Toll-like receptor 2 mediates prostaglandin E2 production in murine peritoneal macrophages and splenocytes in response to Candida albicans. Res. Microbiol. 156, 115–118 (2005).
Tada, H. et al. Saccharomyces cerevisiae- and Candida albicans-derived mannan induced production of tumor necrosis factor α by human monocytes in a CD14- and Toll-like receptor 4-dependent manner. Microbiol. Immunol. 46, 503–512 (2002).
Stahl, P.D. & Ezekowitz, R.A. The mannose receptor is a pattern recognition receptor involved in host defense. Curr. Opin. Immunol. 10, 50–55 (1998).
Lee, S.J., Zheng, N.Y., Clavijo, M. & Nussenzweig, M.C. Normal host defense during systemic candidiasis in mannose receptor-deficient mice. Infect. Immun. 71, 437–445 (2003).
Swain, S.D., Lee, S.J., Nussenzweig, M.C. & Harmsen, A.G. Absence of the macrophage mannose receptor in mice does not increase susceptibility to Pneumocystis carinii infection in vivo. Infect. Immun. 71, 6213–6221 (2003).
Ohno, N., Miura, N.N., Nakajima, M. & Yadomae, T. Antitumor 1,3-β-glucan from cultured fruit body of Sparassis crispa. Biol. Pharm. Bull. 23, 866–872 (2000).
Di Carlo, F.J. & Fiore, J.V. On the composition of zymosan. Science 127, 756–757 (1958).
Kopp, E.B. & Medzhitov, R. The Toll-receptor family and control of innate immunity. Curr. Opin. Immunol. 11, 13–18 (1999).
Gantner, B.N., Simmons, R.M., Canavera, S.J., Akira, S. & Underhill, D.M. Collaborative induction of inflammatory responses by dectin-1 and Toll-like receptor 2. J. Exp. Med. 197, 1107–1117 (2003).
Brown, G.D. et al. Dectin-1 mediates the biological effects of β-glucans. J. Exp. Med. 197, 1119–1124 (2003).
Underhill, D.M. et al. The Toll-like receptor 2 is recruited to macrophage phagosomes and discriminates between pathogens. Nature 401, 811–815 (1999).
Kawai, T., Adachi, O., Ogawa, T., Takeda, K. & Akira, S. Unresponsiveness of MyD88-deficient mice to endotoxin. Immunity 11, 115–122 (1999).
Hida, S., Nagi-Miura, N., Adachi, Y. & Ohno, N. Beta-glucan derived from zymosan acts as an adjuvant for collagen-induced arthritis. Microbiol. Immunol. 50, 453–461 (2006).
Pollock, J.D. et al. Mouse model of X-linked chronic granulomatous disease, an inherited defect in phagocyte superoxide production. Nat. Genet. 9, 202–209 (1995).
Edwards, A.D. et al. Microbial recognition via Toll-like receptor-dependent and -independent pathways determines the cytokine response of murine dendritic cell subsets to CD40 triggering. J. Immunol. 169, 3652–3660 (2002).
Smits, G.J., Kapteyn, J.C., van den Ende, H. & Klis, F.M. Cell wall dynamics in yeast. Curr. Opin. Microbiol. 2, 348–352 (1999).
Hanano, R., Reifenberg, K. & Kaufmann, S.H. Activated pulmonary macrophages are insufficient for resistance against Pneumocystis carinii. Infect. Immun. 66, 305–314 (1998).
Rudmann, D.G., Preston, A.M., Moore, M.W. & Beck, J.M. Susceptibility to Pneumocystis carinii in mice is dependent on simultaneous deletion of IFN-γ and type 1 and 2 TNF receptor genes. J. Immunol. 161, 360–366 (1998).
Rogers, N.C. et al. Syk-dependent cytokine induction by dectin-1 reveals a novel pattern recognition pathway for C type lectins. Immunity 22, 507–517 (2005).
Mansour, M.K. & Levitz, S.M. Interactions of fungi with phagocytes. Curr. Opin. Microbiol. 5, 359–365 (2002).
Netea, M.G. et al. Immune sensing of Candia albicans require cooperative recognition of mannans and glucans by lectin and Toll-like receptors. J. Clin. Invest. 116, 1642–1650 (2006).
Adachi, O. et al. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity 9, 143–150 (1998).
Harada, T. et al. IFN-gamma induction by SCG, 1,3-β-D-glucan from Sparassis crispa, in DBA/2 mice in vitro. J. Interferon Cytokine Res. 22, 1227–1239 (2002).
Saijo, S., Asano, M., Horai, R., Yamamoto, H. & Iwakura, Y. Suppression of autoimmune arthritis in interleukin-1-deficient mice in which T cell activation is impaired due to low levels of CD40 ligand and OX40 expression on T cells. Arthritis Rheum. 46, 533–544 (2002).
Ishibashi, K. et al. Relationship between the physical properties of Candida albicans cell well β-glucan and activation of leukocytes in vitro. Int. Immunopharmacol. 2, 1109–1122 (2002).
Shinkai, Y. et al. Restoration of T cell development in RAG-2-deficient mice by functional TCR transgenes. Science 259, 822–825 (1993).
Habu, K. et al. The human T cell leukemia virus type I-tax gene is responsible for the development of both inflammatory polyarthropathy resembling rheumatoid arthritis and noninflammatory ankylotic arthropathy in transgenic mice. J. Immunol. 162, 2956–2963 (1999).
Nakae, S., Asano, M., Horai, R. & Iwakura, Y. Interleukin-1β, but not interleukin-1α, is required for T-cell-dependent antibody production. Immunology 104, 402–409 (2001).
Nakae, S. et al. Antigen-specific T cell sensitization is impaired in IL-17-deficient mice, causing suppression of allergic cellular and humoral responses. Immunity 17, 375–387 (2002).
Furuta, T., Ueda, K., Kyuwa, S. & Fujiwara, K. Effect of T-cell transfer on Pneumocystis carinii infection in nude mice. Jpn. J. Exp. Med. 54, 57–64 (1984).
Furuta, T. et al. Therapeutic effects of water-soluble echinocandin compounds on Pneumocystis pneumonia in mice. Antimicrob. Agents Chemother. 42, 37–39 (1998).
Acknowledgements
DO11.10 mice were provided by D.Y. Loh (Washington University School of Medicine). Supported by Grants-in-Aid from the Ministry of Education, Culture, Sports, Science, and Technology of Japan and the Ministry of Health and Welfare of Japan (Y.I. and S.S.) and by the Japan Society for the Promotion of Science (N.F.).
Author information
Authors and Affiliations
Contributions
S.S. mainly contributed throughout this work in collaboration with N.F., S.C. and K. Seki; H.K. and K. Sudo did embryonic stem cell culture and produced chimeric mice; Y.A. and N.O. made β-glucan preparations and did cytokine production experiments; T.F. did in vivo P. carinii experiments and provided P. carinii for the in vitro experiments; T.K., K.N. and K.K. did in vivo C. albicans experiments; S.A. provided Myd88−/− mice; and Y.I. supervised the study, designed the experiments and edited the draft paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
Generation of dectin-1-deficient mice. (PDF 147 kb)
Supplementary Fig. 2
Survival and fungal burdens of C. albicans–infected mice. (PDF 354 kb)
Rights and permissions
About this article
Cite this article
Saijo, S., Fujikado, N., Furuta, T. et al. Dectin-1 is required for host defense against Pneumocystis carinii but not against Candida albicans. Nat Immunol 8, 39–46 (2007). https://doi.org/10.1038/ni1425
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni1425
This article is cited by
-
Blocking Dectin-1 prevents colorectal tumorigenesis by suppressing prostaglandin E2 production in myeloid-derived suppressor cells and enhancing IL-22 binding protein expression
Nature Communications (2023)
-
Phagosomal signalling of the C-type lectin receptor Dectin-1 is terminated by intramembrane proteolysis
Nature Communications (2022)
-
The Genetics of Eczema Herpeticum
Clinical Reviews in Allergy & Immunology (2022)
-
Mint3 depletion-mediated glycolytic and oxidative alterations promote pyroptosis and prevent the spread of Listeria monocytogenes infection in macrophages
Cell Death & Disease (2021)
-
TREM2 is a receptor for non-glycosylated mycolic acids of mycobacteria that limits anti-mycobacterial macrophage activation
Nature Communications (2021)