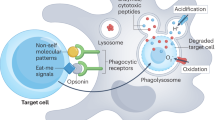
Abstract
The present paradigm dictates that phagocytosis is accomplished mainly by 'professional' phagocytes (such as macrophages and monocytes), whereas B cells lack phagocytic capabilities. Here we demonstrate that B cells from teleost fish have potent in vitro and in vivo phagocytic activities. Particle uptake by B cells induced activation of 'downstream' degradative pathways, leading to 'phagolysosome' formation and intracellular killing of ingested microbes. Those results indicate a previously unknown function for B cells in the innate immunity of these primitive animals. A considerable proportion of Xenopus laevis B cells were also phagocytic. Our findings support the idea that B cells evolved from an ancestral phagocytic cell type and provide an evolutionary framework for understanding the close relationship between mammalian B lymphocytes and macrophages.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Desjardins, M., Houde, M. & Gagnon, E. Phagocytosis: the convoluted way from nutrition to adaptive immunity. Immunol. Rev. 207, 158–165 (2005).
Aderem, A. & Underhill, D.M. Mechanisms of phagocytosis in macrophages. Annu. Rev. Immunol. 17, 593–623 (1999).
Stuart, L.M. & Ezekowitz, R.A. Phagocytosis: elegant complexity. Immunity 22, 539–550 (2005).
Rabinovitch, M. Professional and non-professional phagocytes: an introduction. Trends Cell Biol. 5, 85–87 (1995).
Vidard, L. et al. Analysis of MHC class II presentation of particulate antigens of B lymphocytes. J. Immunol. 156, 2809–2818 (1996).
Ochando, J.C. et al. Alloantigen-presenting plasmacytoid dendritic cells mediate tolerance to vascularized grafts. Nat. Immunol. 7, 652–662 (2006).
Boyd, A.W. & Schrader, J.W. Derivation of macrophage-like lines from the pre-B lymphoma ABLS 8.1 using 5-azacytidine. Nature 297, 691–693 (1982).
Martin, M. et al. A novel cellular model (SPGM 1) of switching between the pre-B cell and myelomonocytic lineages. J. Immunol. 150, 4395–4406 (1993).
Tanaka, T., Wu, G.E. & Paige, C.J. Characterization of the B cell-macrophage lineage transition in 70Z/3 cells. Eur. J. Immunol. 24, 1544–1548 (1994).
Cumano, A., Paige, C.J., Iscove, N.N. & Brady, G. Bipotential precursors of B cells and macrophages in murine fetal liver. Nature 356, 612–615 (1992).
Montecino-Rodriguez, E., Leathers, H. & Dorshkind, K. Bipotential B-macrophage progenitors are present in adult bone marrow. Nat. Immunol. 2, 83–88 (2001).
Iwama, G. & Nakanishi, T. in Fish Physiology Series 63–103 (Academic, San Diego, 1997).
Zapata, A. & Amemiya, C.T. Phylogeny of lower vertebrates and their immunological structures. Curr. Top. Microbiol. Immunol. 248, 67–107 (2000).
Miller, N. et al. Functional and molecular characterization of teleost leukocytes. Immunol. Rev. 166, 187–197 (1998).
Rombout, J.H., Huttenhuis, H.B., Picchietti, S. & Scapigliati, G. Phylogeny and ontogeny of fish leucocytes. Fish Shellfish Immunol. 19, 441–455 (2005).
Hansen, J.D., Landis, E.D. & Phillips, R.B. Discovery of a unique Ig heavy-chain isotype (IgT) in rainbow trout: implications for a distinctive B cell developmental pathway in teleost fish. Proc. Natl. Acad. Sci. USA 102, 6919–6924 (2005).
Danilova, N., Bussmann, J., Jekosch, K. & Steiner, L.A. The immunoglobulin heavy-chain locus in zebrafish: identification and expression of a previously unknown isotype, immunoglobulin Z. Nat. Immunol. 6, 295–302 (2005).
Solem, S.T. & Stenvik, J. Antibody repertoire development in teleosts-a review with emphasis on salmonids and Gadus morhua L. Dev. Comp. Immunol. 30, 57–76 (2006).
Flajnik, M.F. & Rumfelt, L.L. Early and natural antibodies in non-mammalian vertebrates. Curr. Top. Microbiol. Immunol. 252, 233–240 (2000).
Jansson, E. et al. Monoclonal antibodies to lymphocytes of rainbow trout (Oncorhynchus mykiss). Fish Shellfish Immunol. 14, 239–257 (2003).
Miyadai, T., Ootani, M., Tahara, D., Aoki, M. & Saitoh, K. Monoclonal antibodies recognising serum immunoglobulins and surface immunoglobulin-positive cells of puffer fish, torafugu (Takifugu rubripes). Fish Shellfish Immunol. 17, 211–222 (2004).
Secombes, C.J. & Fletcher, T.C. The role of phagocytes in the protective mechanisms of fish. Ann. Rev. Fish Dis. 2, 53–71 (1992).
Flajnik, M.F. & Du Pasquier, L. Evolution of innate and adaptive immunity: can we draw a line? Trends Immunol. 25, 640–644 (2004).
DeLuca, D., Wilson, M. & Warr, G.W. Lymphocyte heterogeneity in the trout, Salmo gairdneri, defined with monoclonal antibodies to IgM. Eur. J. Immunol. 13, 546–551 (1983).
Andersson, E. & Matsunaga, T. Complete cDNA sequence of a rainbow trout IgM gene and evolution of vertebrate IgM constant domains. Immunogenetics 38, 243–250 (1993).
Afonso, A., Ellis, A.E. & Silva, M.T. The leucocyte population of the unstimulated peritoneal cavity of rainbow trout (Oncorhynchus mykiss). Fish Shellfish Immunol. 5, 335–348 (1997).
Honda, T. et al. Molecular cloning and expression analysis of a macrophage-colony stimulating factor receptor-like gene from rainbow trout, Oncorhynchus mykiss. Mol. Immunol. 42, 1–8 (2005).
Chitu, V. & Stanley, E.R. Colony-stimulating factor-1 in immunity and inflammation. Curr. Opin. Immunol. 18, 39–48 (2006).
Barreda, D.R., Hanington, P.C., Stafford, J.L. & Belosevic, M. A novel soluble form of the CSF-1 receptor inhibits proliferation of self-renewing macrophages of goldfish (Carassius auratus L.). Dev. Comp. Immunol. 29, 879–894 (2005).
Wines, B.D., Trist, H.M., Monteiro, R.C., Van Kooten, C. & Hogarth, P.M. Fc receptor γ chain residues at the interface of the cytoplasmic and transmembrane domains affect association with FcαRI, surface expression, and function. J. Biol. Chem. 279, 26339–26345 (2004).
Shen, L. et al. Identification and characterization of clonal NK-like cells from channel catfish (Ictalurus punctatus). Dev. Comp. Immunol. 28, 139–152 (2004).
Boshra, H. et al. Cloning, expression, cellular distribution, and role in chemotaxis of a C5a receptor in rainbow trout: the first identification of a C5a receptor in a nonmammalian species. J. Immunol. 172, 4381–4390 (2004).
Abrahao, T.B., Freymuller, E., Mortara, R.A., Lopes, J.D. & Mariano, M. Morphological characterization of mouse B-1 cells. Immunobiol. 208, 401–411 (2003).
Polliack, A. et al. Identification of human B and T lymphocytes by scanning electron microscopy. J. Exp. Med. 138, 607–624 (1973).
Worth, R.G. et al. The cytoplasmic domain of FcγRIIA (CD32) participates in phagolysosome formation. Blood 98, 3429–3434 (2001).
Morisawa, Y. et al. Lysosomal glycogen storage disease with normal acid maltase with early fatal outcome. J. Neurol. Sci. 160, 175–179 (1998).
Vosbeck, K., James, P.R. & Zimmermann, W. Antibiotic action on phagocytosed bacteria measured by a new method for determining viable bacteria. Antimicrob. Agents Chemother. 25, 735–741 (1984).
Hsu, E. & Du Pasquier, L. Studies on Xenopus immunoglobulins using monoclonal antibodies. Mol. Immunol. 21, 257–270 (1984).
Pier, G.B., Lyczak, J.B. & Wetzler, L.M. in Immunology, Infection and Immunity Ch. 3 (ASM, Washington, DC, 2004).
Mori, M. et al. IL-4 promotes the migration of circulating B cells to the spleen and increases splenic B cell survival. J. Immunol. 164, 5704–5712 (2000).
Fleire, S.J. et al. B cell ligand discrimination through a spreading and contraction response. Science 312, 738–741 (2006).
Davidson, W.F., Pierce, J.H. & Holmes, K.L. Evidence for a developmental relationship between CD5+ B-lineage cells and macrophages. Ann. NY Acad. Sci. 651, 112–129 (1992).
Xie, H., Ye, M., Feng, R. & Graf, T. Stepwise reprogramming of B cells into macrophages. Cell 117, 663–676 (2004).
Borrello, M.A. & Phipps, R.P. The B/macrophage cell: an elusive link between CD5+ B lymphocytes and macrophages. Immunol. Today 17, 471–475 (1996).
Graf, B.A. et al. Biphenotypic B/macrophage cells express COX-1 and up-regulate COX-2 expression and prostaglandin E2 production in response to pro-inflammatory signals. Eur. J. Immunol. 29, 3793–3803 (1999).
Endo, Y. et al. Two lineages of mannose-binding lectin-associated serine protease (MASP) in vertebrates. J. Immunol. 161, 4924–4930 (1998).
Katsura, Y. Redefinition of lymphoid progenitors. Nat. Rev. Immunol. 2, 127–132 (2002).
Plytycz, B. & Seljelid, R. Dual origin of lymphocytes? Immunol. Today 20, 53–55 (1999).
Kawamoto, H. A close developmental relationship between the lymphoid and myeloid lineages. Trends Immunol. 27, 169–175 (2006).
Boshra, H. et al. Characterization of a C3a receptor in rainbow trout and Xenopus: the first identification of C3a receptors in nonmammalian species. J. Immunol. 175, 2427–2437 (2005).
Miller, N.W. et al. Development and characterization of channel catfish long term B cell lines. J. Immunol. 152, 2180–2189 (1994).
Li, J., Peters, R., LaPatra, S.E., Vazzana, M. & Sunyer, J.O. Anaphylatoxin-like molecules generated during complement activation induce a dramatic enhancement of particle uptake in rainbow trout phagocytes. Dev. Comp. Immunol. 28, 1005–1021 (2004).
Miller, N.W., Bly, J.E., van Ginkel, F., Ellsaesser, C.F. & Clem, L.W. Phylogeny of lymphocyte heterogeneity: identification and separation of functionally distinct subpopulations of channel catfish lymphocytes with monoclonal antibodies. Dev. Comp. Immunol. 11, 739–747 (1987).
Acknowledgements
We thank X. Zhao (Biomedical Imaging Core, School of Medicine, University of Pennsylvania), R. Meade and N. Shah (Biomedical Imaging Core, School of Medicine, University of Pennsylvania) for technical assistance; and A. Bhandoola, L. Bello, P. Scott and A. Thomas-Tikhonenko for critical reading of the manuscript. Supported by the National Science Foundation (MCB-0417078 to J.O.S.) and the National Research Initiative of the US Department of Agriculture Cooperative State Research, Education and Extension Service (2004-01599 to J.O.S.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
Characterization of trout B cells. (PDF 180 kb)
Supplementary Fig. 2
Phagocytic IgM+ cells are non-adherent. (PDF 105 kb)
Supplementary Fig. 3
Catfish blood B cells are phagocytic. (PDF 2149 kb)
Supplementary Fig. 4
Amino acid sequence alignment. (PDF 148 kb)
Supplementary Fig. 5
Ultrastructural features of bead uptake by trout phagocytic IgM+ cells. (PDF 166 kb)
Supplementary Fig. 6
In vivo analysis of phagocytosis by peritoneal cavity trout IgM+ and IgM− cells. (PDF 90 kb)
Supplementary Table 1
Primers used for RT-PCR. (PDF 45 kb)
Rights and permissions
About this article
Cite this article
Li, J., Barreda, D., Zhang, YA. et al. B lymphocytes from early vertebrates have potent phagocytic and microbicidal abilities. Nat Immunol 7, 1116–1124 (2006). https://doi.org/10.1038/ni1389
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni1389
This article is cited by
-
Effectiveness of a proteoliposome-based vaccine against salmonid rickettsial septicaemia in Oncorhynchus mykiss
Veterinary Research (2021)
-
Evaluation of an in vitro assay to screen for the immunotoxic potential of chemicals to fish
Scientific Reports (2021)
-
Identification of genetic loci associated with higher resistance to pancreas disease (PD) in Atlantic salmon (Salmo salar L.)
BMC Genomics (2020)
-
Aeromonas salmonicida activates rainbow trout IgM+ B cells signalling through Toll like receptors
Scientific Reports (2020)