Abstract
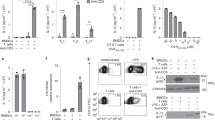
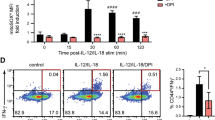
T cell receptor (TCR) stimulation induces rapid generation of reactive oxygen species, although the mechanisms for this are unclear. Here we found that T cells expressed a functional phagocyte-type nicotinamide adenine dinucleotide phosphate (NADPH) oxidase. TCR crosslinking induced oxidase activation through the recruitment of preformed Fas ligand and Fas. TCR stimulation induced three separable events generating reactive oxygen species: rapid hydrogen peroxide production independent of Fas or NADPH oxidase; sustained hydrogen peroxide production dependent on both Fas and NADPH oxidase; and delayed superoxide production that was dependent on Fas ligand and Fas yet independent of NADPH oxidase. NADPH oxidase–deficient T cells showed enhanced activation of the kinase Erk and a relative increase in T helper type 1 cytokine secretion. Thus, mature T cells express a phagocyte-type NADPH oxidase that regulates elements of TCR signaling.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Babior, B.M. NADPH oxidase: an update. Blood 93, 1464–1476 (1999).
Segal, B.H., Leto, T.L., Gallin, J.I., Malech, H.L. & Holland, S.M. Genetic, biochemical, and clinical features of chronic granulomatous disease. Medicine (Baltimore) 79, 170–200 (2000).
Pollock, J.D. et al. Mouse model of X-linked chronic granulomatous disease, an inherited defect in phagocyte superoxide production. Nat. Genet. 9, 202–209 (1995).
Jackson, S.H., Gallin, J.I. & Holland, S.M. The p47phox mouse knock-out model of chronic granulomatous disease. J. Exp. Med. 182, 751–758 (1995).
Yang, S., Panoskaltsis-Mortari, A., Shukla, M., Blazar, B.R. & Haddad, I.Y. Exuberant inflammation in nicotinamide adenine dinucleotide phosphate-oxidase-deficient mice after allogeneic marrow transplantation. J. Immunol. 168, 5840–5847 (2002).
Nathan, C. & Shiloh, M.U. Reactive oxygen and nitrogen intermediates in the relationship between mammalian hosts and microbial pathogens. Proc. Natl. Acad. Sci. USA 97, 8841–8848 (2000).
Ushio-Fukai, M. et al. Reactive oxygen species mediate the activation of Akt/protein kinase B by angiotensin II in vascular smooth muscle cells. J. Biol. Chem. 274, 22699–226704 (1999).
Bae, Y.S. et al. Epidermal growth factor (EGF)-induced generation of hydrogen peroxide. Role in EGF receptor-mediated tyrosine phosphorylation. J. Biol. Chem. 272, 217–221 (1997).
Sundaresan, M., Yu, Z.X., Ferrans, V.J., Irani, K. & Finkel, T. Requirement for generation of H2O2 for platelet-derived growth factor signal transduction. Science 270, 296–299 (1995).
Lee, S.L., Wang, W.W., Finlay, G.A. & Fanburg, B.L. Serotonin stimulates mitogen-activated protein kinase activity through the formation of superoxide anion. Am. J. Physiol. 277, L282–L291 (1999).
Lo, Y.Y.C., Wong, J.M.S. & Cruz, T.F. Reactive oxygen species mediate cytokine activation of c-Jun NH2-terminal kinases. J. Biol. Chem. 271, 15703–15707 (1996).
Krieger-Brauer, H.I. & Kather, H. The stimulus-sensitive H2O2-generating system present in human fat-cell plasma membranes is multireceptor-linked and under antagonistic control by hormones and cytokines. Biochem. J. 307, 543–548 (1995).
Lee, J.R. & Koretzky, G.A. Production of reactive oxygen intermediates following CD40 ligation correlates with c-Jun N-terminal kinase activation and IL-6 secretion in murine B lymphocytes. Eur. J. Immunol. 28, 4188–4197 (1998).
Sundaresan, M. et al. Regulation of reactive-oxygen-species generation in fibroblasts by Rac1. Biochem. J. 318, 379–382 (1996).
Jayanthi, S., Ordonez, S., McCoy, M.T. & Cadet, J.L. Dual mechanism of Fas-induced cell death in neuroglioma cells: a role for reactive oxygen species. Brain Res. Mol. Brain Res. 72, 158–165 (1999).
Condino-Neto, A. & Newburger, P.E. NADPH oxidase activity and cytochrome b558 content of human Epstein-Barr-virus-transformed B lymphocytes correlate with expression of genes encoding components of the oxidase system. Arch. Biochem. Biophys. 360, 158–164 (1998).
Frey, R.S., Rahman, A., Kefer, J.C., Minshall, R.D. & Malik, A.B. PKCz regulates TNF-a-induced activation of NADPH oxidase in endothelial cells. Circ. Res. 90, 1012–1019 (2002).
Bendall, J.K., Cave, A.C., Heymes, C., Gall, N. & Shah, A.M. Pivotal role of a gp91phox-containing NADPH oxidase in angiotensin II-induced cardiac hypertrophy in mice. Circulation 105, 293–296 (2002).
Griendling, K.K., Sorescu, D. & Ushio-Fukai, M. NAD(P)H oxidase: role in cardiovascular biology and disease. Circ. Res. 86, 494–501 (2000).
Lassegue, B. et al. Novel gp91phox homologues in vascular smooth muscle cells: Nox1 mediates angiotensin II-induced superoxide formation and redox-sensitive signaling pathways. Circ. Res. 88, 888–894 (2001).
Banfi, B., Clark, R.A., Steger, K. & Krause, K.H. Two novel proteins activate superoxide generation by the NADPH oxidase NOX1. J. Biol. Chem. 278, 3510–3513 (2003).
Devadas, S., Zaritskaya, L., Rhee, S.G., Oberley, L. & Williams, M.S. Discrete generation of superoxide and hydrogen peroxide by T cell receptor stimulation: selective regulation of mitogen-activated protein kinase activation and fas ligand expression. J. Exp. Med. 195, 59–70 (2002).
van Reyk, D.M., King, N.J., Dinauer, M.C. & Hunt, N.H. The intracellular oxidation of 2′,7′-dichlorofluorescein in murine T lymphocytes. Free Radic. Biol. Med. 30, 82–88 (2001).
Kwon, J., Devadas, S. & Williams, M.S. T cell receptor-stimulated generation of hydrogen peroxide inhibits MEK-ERK activation and lck serine phosphorylation. Free Radic. Biol. Med. 35, 406–417 (2003).
Williams, M.S. & Henkart, P.A. The role of reactive oxygen intermediates in TCR-induced death of T cell blasts and hybridomas. J. Immunol. 157, 2395–2402 (1996).
Hasebe, T., Someya, A. & Nagaoka, I. Identification of a splice variant mRNA of p40phox, an NADPH oxidase component of phagocytes. FEBS Lett. 455, 257–261 (1999).
Lomax, K.L., Leto, T.L., Nunoi, H., Gallin, J.I. & Malech, H.L. Recombinant 47-kilodalton cytosol factor restores NADPH oxidase in chronic granulomatous disease. Science 245, 409–412 (1989).
Emmendorffer, A. et al. Evaluation of flow cytometric methods for diagnosis of chronic granulomatous disease variants under routine laboratory conditions. Cytometry 18, 147–155 (1994).
Yamauchi, A. et al. Location of the epitope for 7D5, a monoclonal antibody raised against human flavocytochrome b558, to the extracellular peptide portion of primate gp91phox. Microbiol. Immunol. 45, 249–257 (2001).
Li, J.M. & Shah, A.M. Intracellular localization and preassembly of the NADPH oxidase complex in cultured endothelial cells. J. Biol. Chem. 277, 19952–19960 (2002).
Maly, F.E. et al. Superoxide-dependent nitroblue tetrazolium reduction and expression of cytochrome b-245 components by human tonsillar B lymphocytes and B cell lines. J. Immunol. 142, 1260–1267 (1989).
Bossi, G. & Griffiths, G.M. Degranulation plays an essential part in regulating cell surface expression of Fas ligand in T cells and natural killer cells. Nat. Med. 5, 90–96 (1999).
Martinez-Lorenzo, M.J. et al. Activated human T cells release bioactive Fas ligand and APO2 ligand in microvesicles. J. Immunol. 163, 1274–1281 (1999).
Zhang, J. et al. Regulation of fas ligand expression during activation-induced cell death in T cells by p38 mitogen-activated protein kinase and c-Jun NH2-terminal kinase. J. Exp. Med. 191, 1017–1030 (2000).
Ju, S.T. et al. Fas (CD95)/FasL interactions required for programmed cell death after T-cell activation. Nature 373, 444–448 (1995).
Williams, M.S. & Kwon, J. T cell receptor stimulation, reactive oxygen species and cell signaling. Free Radic. Biol. Med. advance online publication, 20 June 2004 (do:10.016/j.freeradbiomed.2004.05.029).
Suzuki, Y., Ono, Y. & Hirabayashi, Y. Rapid and specific reactive oxygen species generation via NADPH oxidase activation during Fas-mediated apoptosis. FEBS Lett. 425, 209–212 (1998).
Tsunawaki, S. & Yoshikawa, K. Relationships of p40phox with p67phox in the activation and expression of the human respiratory burst NADPH oxidase. J. Biochem. (Tokyo) 128, 777–783 (2000).
Krieger-Brauer, H.I. & Kather, H. Human fat cells possess a plasma membrane-bound H2O2-generating system that is activated by insulin via a mechanism bypassing the receptor kinase. J. Clin. Invest. 89, 1006–1013 (1992).
Thannickal, V.J. & Fanburg, B.L. Activation of an H2O2-generating NADH oxidase in human lung fibroblasts by transforming growth factor b1. J. Biol. Chem. 270, 30334–30338 (1995).
Mahadev, K. et al. The NAD(P)H oxidase homolog Nox4 modulates insulin-stimulated generation of H2O2 and plays an integral role in insulin signal transduction. Mol. Cell Biol. 24, 1844–1854 (2004).
Geiszt, M., Witta, J., Baffi, J., Lekstrom, K. & Leto, T.L. Dual oxidases represent novel hydrogen peroxide sources supporting mucosal surface host defense. FASEB J. 17, 1502–1504 (2003).
Leavey, P.J. et al. A 29-kDa protein associated with p67phox expresses both peroxiredoxin and phospholipase A2 activity and enhances superoxide anion production by a cell-free system of NADPH oxidase activity. J. Biol. Chem. 277, 45181–45187 (2002).
Lambeth, J.D. Nox enzymes and the biology of reactive oxygen. Nat. Rev. Immunol. 4, 181–189 (2004).
Esposti, M.D., Hatzinisiriou, I., McLennan, H. & Ralph, S. Bcl-2 and mitochondrial oxygen radicals. New approaches with reactive oxygen species-sensitive probes. J. Biol. Chem. 274, 29831–29837 (1999).
Banfi, B. et al. A Ca2+-activated NADPH oxidase in testis, spleen, and lymph nodes. J. Biol. Chem. 276, 37594–37601 (2001).
Ha, Y.J. & Lee, J.R. Role of TNF receptor-associated factor 3 in the CD40 signaling by production of reactive oxygen species through association with p40phox, a cytosolic subunit of nicotinamide adenine dinucleotide phosphate oxidase. J. Immunol. 172, 231–239 (2004).
Martinez-Lorenzo, M.J., Alava, M.A., Anel, A., Pineiro, A. & Naval, J. Release of preformed Fas ligand in soluble form is the major factor for activation-induced death of Jurkat T cells. Immunology 89, 511–517 (1996).
Nagata, S. & Suda, T. Fas and Fas ligand: lpr and gld mutations. Immunol. Today 16, 39–43 (1995).
Seshiah, P.N. et al. Angiotensin II stimulation of NAD(P)H oxidase activity: upstream mediators. Circ. Res. 91, 406–413 (2002).
Jorritsma, P.J., Brogdon, J.L. & Bottomly, K. Role of TCR-induced extracellular signal-regulated kinase activation in the regulation of early IL-4 expression in naive CD4+ T cells. J. Immunol. 170, 2427–2434 (2003).
Segal, B.H. et al. The p47phox−/− mouse model of chronic granulomatous disease has normal granuloma formation and cytokine responses to Mycobacterium avium and Schistosoma mansoni eggs. Infect. Immun. 67, 1659–1665 (1999).
Acknowledgements
We thank P. Henkart and D. Scott for critical reading of the manuscript. Supported by the American Heart Association (0030033N) and American Red Cross.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
Effect of an inhibitor of nitric oxide synthase (L-NMMA) on TCR stimulated ROS generation. (PDF 40 kb)
Supplementary Fig. 2
DCFDA and DHE oxidation occurs in T cells. (PDF 67 kb)
Supplementary Fig. 3
Purity of T cell blast preparations. (PDF 114 kb)
Supplementary Fig. 4
Specific expression of NADPH oxidase component message by murine CD4+ T cell blasts. (PDF 79 kb)
Supplementary Fig. 5
Role of FasL-Fas interactions in anti-CD3 stimulated generation of reactive oxygen species in human T blasts. (PDF 45 kb)
Rights and permissions
About this article
Cite this article
Jackson, S., Devadas, S., Kwon, J. et al. T cells express a phagocyte-type NADPH oxidase that is activated after T cell receptor stimulation. Nat Immunol 5, 818–827 (2004). https://doi.org/10.1038/ni1096
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni1096
This article is cited by
-
Hypoxia exacerbates intestinal injury and inflammatory response mediated by myeloperoxidase during Salmonella Typhimurium infection in mice
Gut Pathogens (2023)
-
STAT proteins in cancer: orchestration of metabolism
Nature Reviews Cancer (2023)
-
Oxidative stress and neuroimmune proteins in a mouse model of autism
Cell Stress and Chaperones (2023)
-
Endothelial dysfunction as a factor leading to arterial hypertension
Pediatric Nephrology (2023)
-
Peripheral T Cell Populations are Differentially Affected in Familial Mediterranean Fever, Chronic Granulomatous Disease, and Gout
Journal of Clinical Immunology (2023)