Abstract
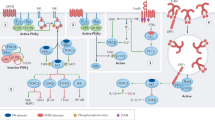
Members of the phosphoinositide-3 kinase (PI3K) family control several cellular responses including cell growth, survival, cytoskeletal remodeling and the trafficking of intracellular organelles in many different types of cell. In particular PI3K has important functions in the immune system. It has been difficult to evaluate the roles of distinct PI3Ks in cellular immune responses because no PI3K inhibitors are specific for individual family members and because most stimuli activate several PI3K enzymes. The development of gene-targeted mice now enables us to examine the physiological functions of individual PI3K enzymes in the immune system in vivo.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Kane, L.P., Lin, J. & Weiss, A. Signal transduction by the TCR for antigen. Curr. Opin. Immunol. 12, 242–249 (2000).
Kurosaki, T. Regulation of B-cell signal transduction by adaptor proteins. Nat. Rev. Immunol. 2, 354–363 (2002),
Kinet, J.P. The high-affinity IgE receptor (FcεRI): from physiology to pathology. Annu. Rev. Immunol. 17, 931–972 (1999).
Wymann, M.P. & Pirola, L. Structure and function of phosphoinositide 3-kinases. Biochim. Biophys. Acta 1436, 127–150 (1998).
Fruman, D.A., Meyers, R.E. & Cantley, L.C. Phosphoinositide kinases. Annu. Rev. Biochem. 67, 481–507 (1998).
Vanhaesebroeck, B. et al. Synthesis and function of 3-phosphorylated inositol lipids. Annu. Rev. Biochem. 70, 535–602 (2001).
Katso, R. et al. Cellular function of phosphoinositide 3-kinases: implications for development, homeostasis, and cancer. Annu. Rev. Cell Dev. Biol. 17, 615–675 (2001).
Walker, E.H., Perisic, O., Ried, C., Stephens, L. & Williams, R.L. Structural insights into phosphoinositide 3-kinase catalysis and signalling. Nature 402, 313–320 (1999).
Pacold, M.E. et al. Crystal structure and functional analysis of Ras binding to its effector phosphoinositide 3-kinase γ. Cell 103, 931–943 (2000).
Okkenhaug, K. & Vanhaesebroeck, B. New responsibilities for the PI3K regulatory subunit p85α. Science's STKE 65, 1–5 (2001). http://stke.sciencemag.org/cgi/content/full/sigtrans;2001/65/pe1
Cheever M.L. et al. Phox domain interaction with PtdIns3 targets the Vam7 t-SNARE to vacuole membranes. Nat. Cell Biol. 3, 613–618 (2002).
Xu, Y., Hortsman, H., Seet, L., Wong, S.H. & Hong, W. SNX3 regulates endosomal function through its PX-domain-mediated interaction with PtdIns3P. Nat. Cell Biol. 3, 658–666 (2002).
Kanai, F. et al. The PX domains of p47phox and p40phox bind to lipid products of PI(3)K. Nat. Cell Biol. 3, 675–678 (2001).
Ellson, C.D. et al. PtdIns3P regulates the neutrophil oxidase complex by binding to the PX domain of p40phox. Nat. Cell Biol. 3, 679–682 (2001).
Didichenko, S.A. & Thelen, M. Phosphatidylinositol 3-kinase C2α contains a nuclear localization sequence and associates with nuclear speckles. J. Biol. Chem. 276, 48135–48142 (2001).
Cantley, L.C. & Neel, B.G. New insights into tumor suppression: PTEN suppresses tumor formation by restraining the phosphoinositide 3-kinase/AKT pathway. Proc. Natl. Acad. Sci. USA 96, 4240–4245 (1999).
Ward, S.G. & Cantrell, D.A. Phosphoinositide 3-kinases in T lymphocyte activation. Curr. Opin. Immunol. 13, 332–338 (2001).
Marshall, A.J., Niiro, H., Yun, T.J. & Clark, E.A. Regulation of B-cell activation and differentiation by the phosphatidylinositol 3-kinase and phospholipase Cγ pathway. Immunol. Rev. 176, 30–46 (2000).
Kurosaki, T. & Okada, T. Regulation of phospholipase C-γ2 and phosphoinositide 3-kinase pathways by adaptor proteins in B lymphocytes. Int. Rev. Immunol. 20, 697–711 (2001).
Turner, H. & Kinet, J.P. Signalling through the high-affinity IgE receptor FcεRI. Nature 402, B24–B30 (1999).
Jiang, K. et al. Pivotal role of phosphoinositide-3 kinase in regulation of cytotoxicity in natural killer cells. Nat. Immunol. 1, 419–425 (2000).
Okkenhaug, K. et al. A point mutation in CD28 distinguishes proliferative signals from survival signals. Nat. Immunol. 2, 325–332 (2001).
Jones, R.G. et al. CD28-dependent activation of protein kinase B/Akt blocks Fas-mediated apoptosis by preventing death-inducing signaling complex assembly. J. Exp. Med. 196, 335–348 (2002).
Harada, Y. et al. Critical requirement for the membrane-proximal cytosolic tyrosine residue for CD28-mediated costimulation in vivo. J. Immunol. 166, 3797–3803 (2001).
Wang, Y. et al. The physiologic role of CD19 cytoplasmic tyrosines. Immunity 17, 501–514 (2002).
Gibbins, J.M. et al. The p85 subunit of phosphatidylinositol 3-kinase associates with the Fc receptor γ-chain and linker for activitor of T cells (LAT) in platelets stimulated by collagen and convulxin. J. Biol. Chem. 273, 34437–34443 (1998).
Okada, T., Maeda, A., Iwamatsu, A., Gotoh, K. & Kurosaki, T. BCAP: the tyrosine kinase substrate that connects B cell receptor to phosphoinositide 3-kinase activation. Immunity 13, 817–827 (2000).
Bone, H. & Williams, N.A. Antigen-receptor cross-linking and lipopolysaccharide trigger distinct phosphoinositide 3-kinase-dependent pathways to NF-κB activation in primary B cells. Int. Immunol. 13, 807–816 (2001).
Andjelic, S. et al. Phosphatidylinositol 3-kinase and NF-κB/Rel are at the divergence of CD40-mediated proliferation and survival pathways. J. Immunol. 165, 3860–3867 (2000).
Ardeshna, K.M., Pizzey, A.R., Devereux, S. & Khwaja, A. The PI3 kinase, p38 SAP kinase, and NF-κB signal transduction pathways are involved in the survival and maturation of lipopolysaccharide-stimulated human monocyte-derived dendritic cells. Blood 96, 1039–1046 (2000).
Arbibe, L. et al. Toll-like receptor 2-mediated NF-κB activation requires a Rac1-dependent pathway. Nat. Immunol. 1, 533–540 (2000).
Fukao, T. et al. PI3K-mediated negative feedback regulation of IL-12 production in DCs. Nat. Immunol. 3, 875–881 (2002).
Ishii, K.J. et al. Potential role of phosphatidylinositol 3 kinase, rather than DNA-dependent protein kinase, in CpG DNA-induced immune activation. J. Exp. Med. 196, 269–274 (2002).
Sasaki, T. et al. Function of PI3Kγ in thymocyte development, T cell activation, and neutrophil migration. Science 287, 1040–1046 (2000).
Li, Z. et al. Roles of PLC-β2 and -β3 and PI3Kγ in chemoattractant-mediated signal transduction. Science 287, 1046–1049 (2000).
Hirsch, E. et al. Central role for G protein-coupled phosphoinositide 3-kinase γ in inflammation. Science 287, 1049–1053 (2000).
Laffargue, M. et al. Phosphoinositide 3-kinase γ is an essential amplifier of mast cell function. Immunity 16, 441–451 (2002).
Ferguson, K.M. et al. Structural basis for discrimination of 3-phosphoinositides by pleckstrin homology domains. Mol. Cell 6, 373–384 (2000).
Satterthwaite, A.B., Li., Z. & Witte, O.N. Btk function in B cell development and response. Semin. Immunol. 10, 309–316 (1998).
Suzuki, H. et al. PI3K and Btk differentially regulate B cell antigen receptor mediated signal transduction. Nat. Immunol. 4, 280–286 (2003).
Okkenhaug, K. et al. Impaired B and T cell antigen receptor signaling in p110δ PI 3-kinase mutant mice. Science 297, 1031–1034 (2002).
Clayton, E. et al. A crucial role for the p110δ subunit of phosphatidylinositol 3-kinase in B cell development and activation. J. Exp. Med. 196, 753–763 (2002).
Jou, S.-T. et al. Essential, nonredundant role for the phosphoinositide 3-kinase p110δ in signaling by the B-cell receptor complex. Mol. Cell. Biol. 22, 8580–8591 (2002).
Walker, E.H. et al. Structural determinants of phosphoinositide 3-kinase inhibition by wortmannin, LY294002, quercetin, myricetin, and staurosporine. Mol. Cell 6, 909–919 (2000).
Bi, L., Okabe, I., Bernard, D.J., Wynshaw-Boris, A. & Nussbaum, R.L. Proliferative defect and embryonic lethality in mice homozygous for a deletion in the p110α subunit of phosphoinositide 3-kinase. J. Biol. Chem. 274, 10963–10968 (1999).
Bi, L., Okabe, I., Bernard, D.J. & Nussbaum, R.L. Early embryonic lethality in mice deficient in the p110β catalytic subunit of PI 3-kinase. Mamm. Genome 13, 169–172 (2002).
Terauchi, Y. et al. Increased insulin sensitivity and hypoglycaemia in mice lacking the p85α subunit of phosphoinositide 3-kinase. Nat. Genet. 21, 230–235 (1999).
Suzuki, H. et al. Xid-like immunodeficiency in mice with disruption of the p85α subunit of phosphoinositide 3-kinase. Science 283, 390–392 (1999).
Fruman, D.A. et al. Impaired B cell development and proliferation in absence of phosphoinositide 3-kinase p85α. Science 283, 393–397 (1999).
Ueki, K. et al. Increased insulin sensitivity in mice lacking p85β subunit of phosphoinositide 3-kinase. Proc. Natl. Acad. Sci. USA 99, 419–424 (2002).
Helgason, C.D. et al. Targeted disruption of SHIP leads to hemopoietic perturbations, lung pathology, and a shortened life span. Genes Dev. 12, 1610–1620 (1998).
Liu, Q. et al. SHIP is a negative regulator of growth factor receptor-mediated PKB/Akt activation and myeloid cell survival. Genes Dev. 13, 786–791 (1999).
Di Cristofano, A., Pesce, B., Cordon-Cardo, C. & Pandolfi, P.P. Pten is essential for embryonic development and tumour suppression. Nat. Genet. 19, 348–355 (1998).
Suzuki, A. et al. High cancer susceptibility and embryonic lethality associated with mutation of the PTEN tumorsuppressor gene in mice. Curr. Biol. 8, 1169–1178 (1998).
Wang, F. et al. Lipid products of PI(3)Ks maintain persistent cell polarity and directed motility in neutrophils. Nat. Cell Biol. 4, 513–518 (2002).
Vieira, O.V. et al. Distinct roles of class I and class III phosphatidylinositol 3-kinases in phagosome formation and maturation. J. Cell Biol. 155, 19–25 (2001).
Siddhanta, U., McIlroy, J., Shah, A., Zhang, Y. & Backer, J.M. Distinct roles for the p110α and hVPS34 phosphatidylinositol 3′-kinases in vesicular trafficking, regulation of the actin cytoskeleton, and mitogenesis. J. Cell Biol. 143, 1647–1659 (1998).
Akira, S., Takeda, K. & Kaisho, T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat. Immunol. 2, 675–680 (2001).
Trinchieri, G. Interleukin-12: a proinflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigen-specific adaptive immunity. Annu. Rev. Immunol. 13, 251–276 (1995).
Frucht, D.M. et al. IFN-γ production by antigen-presenting cells: mechanisms emerge. Trends Immunol. 22, 556–560 (2001).
MacMicking, J., Xie, Q.-W. & Nathan, C. Nitric oxide and macrophage function. Annu. Rev. Immunol. 15, 323–350 (1997).
Guha, M. & Mackman, N. The phosphatidylinositol 3-kinase–Akt pathway limits lipopolysaccharide activation of signaling pathways and expression of inflammatory mediators in human monocytic cells. J. Biol. Chem. 277, 32124–32132 (2002).
Fukao, T. et al. Selective loss of gastrointestinal mast cells and impaired immunity in PI3K-deficient mice. Nat. Immunol. 3, 295–304 (2002).
Kissel, H. et al. Point mutation in kit receptor tyrosine kinase reveals essential roles for kit signaling in spermatogenesis and oogenesis without affecting other kit responses. EMBO J. 19, 1312–1326 (2000).
Gu, H. et al. Essential role for Gab2 in the allergic response. Nature 412, 186–190 (2001).
Nishida, K. et al. Requirement of Gab2 for mast cell development and KitL/c-Kit signaling. Blood 99, 1866–1869 (2002).
Lu-Kuo, J.M., Fruman, D.A., Joyal, D.M., Cantley, L.C. & Katz, H.R. Impaired kit- but not FcεRI-initiated mast cell activation in the absence of phosphoinositide 3-kinase p85α gene products. J. Biol. Chem. 275, 6022–6029 (2000).
Huber, M. et al. The src homology 2-containing inositol phosphatase (SHIP) is the gatekeeper of mast cell degranulation. Proc. Natl. Acad. Sci. USA 95, 11330–11335 (1998).
Pappu, R. et al. Requirement for B cell linker protein (BLNK) in B cell development. Science 286, 1949–1954 (1999).
Leitges, M. et al. Immunodeficiency in protein kinase Cβ-deficient mice. Science 273, 788–791 (1996).
Wang, D. et al. Phospholipase Cγ2 is essential in the functions of B cell and several Fc receptors. Immunity 13, 25–35 (2000).
Tedford, K. et al. Compensation between Vav-1 and Vav-2 in B cell development and antigen receptor signaling. Nat. Immunol. 2, 548–555 (2001).
Engel, P. et al. Abnormal B lymphocyte development, activation, and differentiation in mice that lack or overexpress the CD19 signal transduction molecule. Immunity 3, 39–50 (1995).
Rickert, R.C., Rajewsky, K. & Roes, J. Impairment of T-cell-dependent B-cell responses and B-1 cell development in CD19-deficient mice. Nature 376, 352–355 (1995).
Ono, M. et al. Deletion of SHIP or SHP-1 reveals two distinct pathways for inhibitory signaling. Cell 90, 293–301 (1997).
Liu, Q. et al. The inositol polyphosphate 5-phosphatase ship is a crucial negative regulator of B cell antigen receptor signaling. J. Exp. Med. 188, 1333–1342 (1998).
Dustin, M.L. & Cooper, J.A. The immunological synapse and the actin cytoskeleton: molecular hardware for T cell signaling. Nat. Immunol. 1, 23–29 (2000).
Costello, P.S., Gallagher, M. & Cantrell, D.A. Sustained and dynamic inositol lipid metabolism inside and outside the immunological synapse. Nat. Immunol. 3, 1082–1089 (2002).
Harriague, J. & Bismuth, G. Imaging antigen-induced PI3K activation in T cells. Nat. Immunol. 3, 1090–1096 (2002).
Anzelon, A.N., Wu, H. & Ricker, R.C. Pten inactivation alters peripheral B lymphocyte fate and reconstitutes CD19 function. Nat. Immunol. 4, 287–294 (2003).
Suzuki, A. et al. Critical roles of Pten in B cell homeostasis and immunoglobulin class switch recombination. J. Exp. Med. 197, 657–667 (2003).
Suzuki, A. et al. T cell-specific loss of Pten leads to defects in central and peripheral tolerance. Immunity 14, 523–534 (2001).
Fang, D. & Liu, Y.C. Proteolysis-independent regulation of PI3K by Cbl-b–mediated ubiquitination in T cells. Nat. Immunol. 2, 870–875 (2001).
Ireton, K., Payrastre, B. & Cossart, P. The Listeria monocytogenes protein InlB is an agonist of mammalian phosphoinositide 3-kinase. J. Biol. Chem. 274, 17025–17032 (1999).
Mansell, A., Khelef, N., Cossart, P. & O'Neill, L.A. Internalin B activates nuclear factor-κB via Ras, phosphoinositide 3-kinase, and Akt. J. Biol. Chem. 276, 43597–43603 (2001).
Celli, J., Olivier, M. & Finlay, B.B. Enteropathogenic Escherichia coli mediates antiphagocytosis through the inhibition of PI 3-kinase-dependent pathways. EMBO J. 20, 1245–1258 (2001).
Norris, F.A. et al. SopB, a protein required for virulence of Salmonella dublin, is an inositol phosphate phosphatase. Proc. Natl. Acad. Sci. USA 95, 14057–14059 (1998).
Acknowledgements
I thank T. Kadowaki, Y. Terauchi and many other colleagues for fruitful collaborations, and K. Takatsu, T. Kurosaki, L.K. Clayton and members of my laboratory for valuable discussions. Supported by a Grant-in-Aid for Creative Scientific Research (13GS0015) and a Grant-in-Aid for Scientific Research B (14370116) from the Japan Society for the Promotion of Science, a Grant-in-Aid for Scientific Research on Priority Areas C (13226112, 14021110), a National Grant-in-Aid for the Establishment of a High-Tech Research Center in a private University, a grant for the Promotion of the Advancement of Education and Research in Graduate Schools, and a Scientific Frontier Research Grant from the Ministry of Education, Culture, Sports, Science and Technology, Japan.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Koyasu, S. The role of PI3K in immune cells. Nat Immunol 4, 313–319 (2003). https://doi.org/10.1038/ni0403-313
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/ni0403-313
This article is cited by
-
Harnessing peroxisome proliferator-activated receptor γ agonists to induce Heme Oxygenase-1: a promising approach for pulmonary inflammatory disorders
Cell Communication and Signaling (2024)
-
PI3K pathway mutation predicts an activated immune microenvironment and better immunotherapeutic efficacy in head and neck squamous cell carcinoma
World Journal of Surgical Oncology (2023)
-
The spatial distribution of GPCR and Gβγ activity across a cell dictates PIP3 dynamics
Scientific Reports (2023)
-
PI3K signalling at the intersection of cardio-oncology networks: cardiac safety in the era of AI
Cellular and Molecular Life Sciences (2022)
-
Respiratory exposure to PM2.5 soluble extract induced chronic lung injury by disturbing the phagocytosis function of macrophage
Environmental Science and Pollution Research (2022)