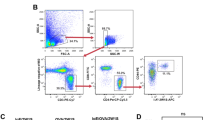
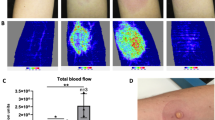
Abstract
Palpable swelling of regional lymph nodes is a common sequela of microbial infections but the mechanism responsible for the sequestration and subsequent coordination of lymphocyte responses within these dynamic structures remains poorly understood. Here we show that draining lymph nodes of mast cell–deficient mice did not demonstrate swelling after intradermal bacterial challenge. Testing of individual mast cell–derived products in this model indicated that tumor necrosis factor was the main mediator of nodal hypertrophy, whereas tryptase and histamine had no effect. After peripheral mast cell activation, both tumor necrosis factor concentrations and the recruitment of circulating T cells were increased within draining nodes. These results show a critical function for peripheral mast cell–derived tumor necrosis factor in regulating the hypertrophy of draining lymph nodes during infection.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Gordon, J.R. & Galli, S.J. Release of both preformed and newly synthesized tumor necrosis factor α (TNF-α)/cachectin by mouse mast cells stimulated via the Fcε RI. A mechanism for the sustained action of mast cell-derived TNF-α during IgE-dependent biological responses. J. Exp. Med. 174, 103–107 (1991).
Gordon, J.R. & Galli, S.J. Mast cells as a source of both preformed and immunologically inducible TNF-α/cachectin. Nature 346, 274–276 (1990).
Zhang, Y., Ramos, B.F. & Jakschik, B.A. Neutrophil recruitment by tumor necrosis factor from mast cells in immune complex peritonitis. Science 258, 1957–1959 (1992).
Finlay-Jones, J.J., Davies, K.V., Sturm, L.P., Kenny, P.A. & Hart, P.H. Inflammatory processes in a murine model of intra-abdominal abscess formation. J. Leukoc. Biol. 66, 583–587 (1999).
Marshall, J.S. & Bienenstock, J. The role of mast cells in inflammatory reactions of the airways, skin and intestine. Curr. Opin. Immunol. 6, 853–859 (1994).
Padawer, J. Mast cells: extended lifespan and lack of granule turnover under normal in vivo conditions. Exp. Mol. Pathol. 20, 269–280 (1974).
Malaviya, R., Ikeda, T., Ross, E. & Abraham, S.N. Mast cell modulation of neutrophil influx and bacterial clearance at sites of infection through TNF-α. Nature 381, 77–80 (1996).
Echtenacher, B., Mannel, D.N. & Hultner, L. Critical protective role of mast cells in a model of acute septic peritonitis. Nature 381, 75–77 (1996).
Malaviya, R. et al. Mast cell phagocytosis of FimH-expressing enterobacteria. J. Immunol. 152, 1907–1914 (1994).
Huang, C. et al. Induction of a selective and persistent extravasation of neutrophils into the peritoneal cavity by tryptase mouse mast cell protease 6. J. Immunol. 160, 1910–1919 (1998).
Huang, C. et al. Evaluation of the substrate specificity of human mast cell tryptase βI and demonstration of its importance in bacterial infections of the lung. J. Biol. Chem. 276, 26276–26284 (2001).
Marone, G., Gentile, M., Petraroli, A., De Rosa, N. & Triggiani, M. Histamine-induced activation of human lung macrophages. Int. Arch. Allergy Immunol. 124, 249–252 (2001).
Burns, A.R. et al. P-selectin mediates neutrophil adhesion to endothelial cell borders. J. Leukoc. Biol. 65, 299–306 (1999).
Jenkins, M.K. et al. In vivo activation of antigen-specific CD4 T cells. Annu. Rev. Immunol. 19, 23–45 (2001).
Banchereau, J. & Steinman, R.M. Dendritic cells and the control of immunity. Nature 392, 245–252 (1998).
Palframan, R.T. et al. Inflammatory chemokine transport and presentation in HEV: a remote control mechanism for monocyte recruitment to lymph nodes in inflamed tissues. J. Exp. Med. 194, 1361–1373 (2001).
Keith, B.R., Maurer, L., Spears, P.A. & Orndorff, P.E. Receptor-binding function of type 1 pili effects bladder colonization by a clinical isolate of Escherichia coli. Infect. Immun. 53, 693–696 (1986).
Baorto, D.M. et al. Survival of FimH-expressing enterobacteria in macrophages relies on glycolipid traffic. Nature 389, 636–639 (1997).
Matzinger, P. The danger model: a renewed sense of self. Science 296, 301–305 (2002).
Tomoe, S., Iwamoto, I., Tomioka, H. & Yoshida, S. Comparison of substance P-induced and compound 48/80-induced neutrophil infiltrations in mouse skin. Int. Arch. Allergy Immunol. 97, 237–242 (1992).
Guo, Y., Hedqvist, P. & Gustafsson, L.E. Absence of mast cell involvement in active systemic anaphylaxis in rats. Eur. J. Pharmacol. 430, 305–310 (2001).
Diaz, B.L. et al. Alloxan diabetes reduces pleural mast cell numbers and the subsequent eosinophil influx induced by allergen in sensitized rats. Int. Arch. Allergy Immunol. 111, 36–43 (1996).
Aridor, M., Traub, L.M. & Sagi-Eisenberg, R. Exocytosis in mast cells by basic secretagogues: evidence for direct activation of GTP-binding proteins. J. Cell Biol. 111, 909–917 (1990).
Getting, S.J. et al. Molecular determinants of monosodium urate crystal-induced murine peritonitis: a role for endogenous mast cells and a distinct requirement for endothelial-derived selectins. J. Pharmacol. Exp. Ther. 283, 123–130 (1997).
McLean, P.G., Ahluwalia, A. & Perretti, M. Association between kinin B1 receptor expression and leukocyte trafficking across mouse mesenteric postcapillary venules. J. Exp. Med. 192, 367–380 (2000).
Cyster, J.G. & Goodnow, C.C. Pertussis toxin inhibits migration of B and T lymphocytes into splenic white pulp cords. J. Exp. Med. 182, 581–586 (1995).
Bargatze, R.F. & Butcher, E.C. Rapid G protein-regulated activation event involved in lymphocyte binding to high endothelial venules. J. Exp. Med. 178, 367–372 (1993).
Warnock, R.A., Askari, S., Butcher, E.C. & von Andrian, U.H. Molecular mechanisms of lymphocyte homing to peripheral lymph nodes. J. Exp. Med. 187, 205–216 (1998).
Berlin-Rufenach, C. et al. Lymphocyte migration in lymphocyte function-associated antigen (LFA)-1-deficient mice. J. Exp. Med. 189, 1467–1478 (1999).
Faveeuw, C., Di Mauro, M.E., Price, A.A. & Ager, A. Roles of α4 integrins/VCAM-1 and LFA-1/ICAM-1 in the binding and transendothelial migration of T lymphocytes and T lymphoblasts across high endothelial venules. Int. Immunol. 12, 241–251 (2000).
Watanabe, C. et al. Spatial heterogeneity of TNF-α-induced T cell migration to colonic mucosa is mediated by MAdCAM-1 and VCAM-1. Am. J. Physiol. Gastrointest. Liver Physiol. 283, G1379–1387 (2002).
Ding, Z., Xiong, K. & Issekutz, T.B. Regulation of chemokine-induced transendothelial migration of T lymphocytes by endothelial activation: differential effects on naive and memory T cells. J. Leukoc. Biol. 67, 825–833 (2000).
Ding, Z., Xiong, K. & Issekutz, T.B. Chemokines stimulate human T lymphocyte transendothelial migration to utilize VLA-4 in addition to LFA-1. J. Leukoc. Biol. 69, 458–466 (2001).
Estess, P., Nandi, A., Mohamadzadeh, M. & Siegelman, M.H. Interleukin 15 induces endothelial hyaluronan expression in vitro and promotes activated T cell extravasation through a CD44-dependent pathway in vivo. J. Exp. Med. 190, 9–19 (1999).
Carlos, T.M. & Harlan, J.M. Leukocyte-endothelial adhesion molecules. Blood 84, 2068–2101 (1994).
van der Poll, T. & van Deventer, S.J. Cytokines and anticytokines in the pathogenesis of sepsis. Infect. Dis. Clin. North Am. 13, 413–426 (1999).
Wang, H.W., Tedla, N., Lloyd, A.R., Wakefield, D. & McNeil, P.H. Mast cell activation and migration to lymph nodes during induction of an immune response in mice. J. Clin. Invest. 102, 1617–1626 (1998).
Robbie-Ryan, M. & Brown, M. The role of mast cells in allergy and autoimmunity. Curr. Opin. Immunol. 14, 728–733 (2002).
Lee, D.M. et al. Mast cells: a cellular link between autoantibodies and inflammatory arthritis. Science 297, 1689–1692 (2002).
Pasparakis, M., Alexopoulou, L., Episkopou, V. & Kollias, G. Immune and inflammatory responses in TNF-α-deficient mice: a critical requirement for TNF-α in the formation of primary B cell follicles, follicular dendritic cell networks and germinal centers, and in the maturation of the humoral immune response. J. Exp. Med. 184, 1397–1411 (1996).
Pasparakis, M. et al. Peyer's patch organogenesis is intact yet formation of B lymphocyte follicles is defective in peripheral lymphoid organs of mice deficient for tumor necrosis factor and its 55-kDa receptor. Proc. Natl. Acad. Sci. USA 94, 6319–6323 (1997).
Acknowledgements
We thank M. Yanagita, R. Goyal, the Duke Photopath Lab and the Duke Human Vaccine Institute Flow Cytometry Core Facility for their assistance with experiments. Supported by funds from the National Institutes of Health and from the Sandler Foundation for Asthma Research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
McLachlan, J., Hart, J., Pizzo, S. et al. Mast cell–derived tumor necrosis factor induces hypertrophy of draining lymph nodes during infection. Nat Immunol 4, 1199–1205 (2003). https://doi.org/10.1038/ni1005
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni1005
This article is cited by
-
Immunomodulatory effect of saponin treatment and microbial infections provoke the expression of Mx gene in catla (Labeo catla)
Aquaculture International (2024)
-
New perspectives on the origins and heterogeneity of mast cells
Nature Reviews Immunology (2023)
-
IgE-activated mast cells enhance TLR4-mediated antigen-specific CD4+ T cell responses
Scientific Reports (2021)
-
Adaptive immune responses to primary and secondary dengue virus infections
Nature Reviews Immunology (2019)
-
Role of Mast Cells in Regulation of T Cell Responses in Experimental and Clinical Settings
Clinical Reviews in Allergy & Immunology (2018)