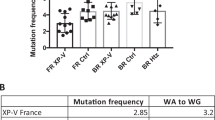
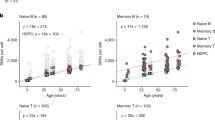
Abstract
Immunoglobulin (Ig) gene hypermutation can be induced in the BL2 Burkitt's lymphoma cell line by IgM cross-linking and coculture with normal or transformed T helper clones. We describe here a T cell–independent in vitro induction assay, by which hypermutation is induced in BL2 cells through simultaneous aggregation of three surface receptors: IgM, CD19 and CD21. The mutations arise as a post-transcriptional event within 90 min. They are stably introduced in the G1 phase of the cell cycle, occurring in one of the two variable gene DNA strands, and eventually become fixed by replication in one of the daughter cells. Inactivation of AID (activation-induced cytidine deaminase) by homologous recombination in BL2 cells completely inhibits the process, thus validating this induction procedure as a model for the in vivo mechanism.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Weigert, M.G., Cesari, I.M., Yonkovich, S.J. & Cohn, M. Variability in the λ light chain sequences of mouse antibody. Nature 228, 1045–1047 (1970).
Wagner, S.D. & Neuberger, M.S. Somatic hypermutation of immunoglobulin genes. Annu. Rev. Immunol 14, 441–457 (1996).
Reynaud, C.A. & Weill, J.C. Postrearrangement diversification processes in gut-associated lymphoid tissues. Curr. Top. Microbiol. Immunol. 212, 7–15 (1996).
Weller, S. et al. CD40-CD40L independent Ig gene hypermutation suggests a second B cell diversification pathway in humans. Proc. Natl. Acad. Sci. USA 98, 1166–1170 (2001).
Muramatsu, M. et al. Class switch recombination and hypermutation require activation-induced cytidine deaminase (AID), a potential RNA editing enzyme. Cell 102, 553–563 (2000).
Revy, P. et al. Activation-induced cytidine deaminase (AID) deficiency causes the autosomal recessive form of the Hyper-IgM syndrome (HIGM2). Cell 102, 565–575 (2000).
Arakawa, H., Hauschild, J. & Buerstedde, J.M. Requirement of the activation-induced deaminase (AID) gene for immunoglobulin gene conversion. Science 295, 1301–1306 (2002).
Harris, R.S., Sale, J.E., Petersen-Mahrt, S.K. & Neuberger, M.S. AID is essential for immunoglobulin V gene conversion in a cultured B cell line. Curr. Biol. 12, 435–438 (2002).
Martin, A. et al. Activation-induced cytidine deaminase turns on somatic hypermutation in hybridomas. Nature 415, 802–806 (2002).
Sale, J.E. & Neuberger, M.S. TdT-accessible breaks are scattered over the immunoglobulin V domain in a constitutively hypermutating B cell line. Immunity 9, 859–869 (1998).
Papavasiliou, F.N. & Schatz, D.G. Cell-cycle-regulated DNA double-stranded breaks in somatic hypermutation of immunoglobulin genes. Nature 408, 216–221 (2000).
Bross, L. et al. DNA double-strand breaks in immunoglobulin genes undergoing somatic hypermutation. Immunity 13, 589–597 (2000).
Kong, Q. & Maizels, N. DNA breaks in hypermutating immunoglobulin genes: evidence for a break-and-repair pathway of somatic hypermutation. Genetics 158, 369–378 (2001).
Peters, A. & Storb, U. Somatic hypermutation of immunoglobulin genes is linked to transcription initiation. Immunity 4, 57–65 (1996).
Goyenechea, B. et al. Cells strongly expressing Igκ transgenes show clonal recruitment of hypermutation: a role for both MAR and the enhancers. EMBO J. 16, 3987–3994 (1997).
Denepoux, S. et al. Induction of somatic mutation in a human B cell line in vitro. Immunity 6, 35–46 (1997).
Zan, H. et al. Induction of Ig somatic hypermutation and class switching in a human monoclonal IgM+ IgD+ B cell line in vitro: definition of the requirements and modalities of hypermutation. J. Immunol. 162, 3437–3447 (1999).
Rogozin, I.B. & Kolchanov, N.A. Somatic hypermutagenesis in immunoglobulin genes. II. Influence of neighbouring base sequence on mutagenesis. Biochim. Biophys. Acta 1171, 11–18 (1992).
Mittrucker, H.W., Muller-Fleckenstein, I., Fleckenstein, B. & Fleischer, B. Herpes virus saimiri-transformed human T lymphocytes: normal functional phenotype and preserved T cell receptor signalling. Int. Immunol. 5, 985–990 (1993).
Poltoratsky, V. et al. Expression of error-prone polymerases in BL2 cells activated for Ig somatic hypermutation. Proc. Natl. Acad. Sci. USA 98, 7976–7981 (2001).
Lazebnik, Y., Kaufmann, S., Desnoyers, S., Poirier, G. & Earnshaw, W. Cleavage of poly(ADP-ribose)polymerase by a proteinase with properties like ICE. Nature 371, 346–347 (1994).
Pasqualucci, L. et al. Hypermutation of multiple proto-oncogenes in B-cell diffuse large-cell lymphomas. Nature 412, 341–346 (2001).
Muto, T., Muramatsu, M., Taniwaki, M., Kinoshita, K. & Honjo, T. Isolation, tissue distribution, and chromosomal localization of the human activation-induced cytidine deaminase (AID) gene. Genomics 68, 85–88 (2000).
Levy et al. Defect in IgV gene somatic hypermutation in Common Variable Immuno-Deficiency Syndrome. Proc. Natl. Acad. Sci. USA 95, 13135–13140 (1998).
Denepoux, S., Fournier, N., Peronne, C., Banchereau, J. & Lebecque, S. T cells can induce somatic mutation in B cell receptor-engaged BL2 Burkitt's lymphoma cells independently of CD40-CD40 ligand interactions. J. Immunol. 164, 1306–1313 (2000).
Fearon, D.T. & Carroll, M.C. Regulation of B lymphocyte responses to foreign and self-antigens by the CD19/CD21 complex. Annu. Rev. Immunol. 18, 393–422 (2000).
Zhang, W. et al. Clonal instability of V region hypermutation in the Ramos Burkitt's lymphoma cell line. Int. Immunol. 13, 1175–1184 (2001).
Rada, C., Jarvis, J.M. & Milstein, C. AID-GFP chimeric protein increases hypermutation of Ig genes with no evidence of nuclear localization. Proc. Natl. Acad. Sci. USA 99, 7003–7008 (2002).
Bertocci, B. et al. Probing immunoglobulin gene hypermutation with microsatellites suggests a nonreplicative short patch DNA synthesis process. Immunity 9, 257–265 (1998).
Papavasiliou, F.N. & Schatz, D.G. The activation-induced deaminase functions in a postcleavage step of the somatic hypermutation process. J. Exp. Med. 195, 1193–1198 (2002).
Bross, L., Muramatsu, M., Kinoshita, K., Honjo, T. & Jacobs . DNA double-strand breaks: prior to but not sufficient in targeting hypermutation. J. Exp. Med. 195, 1187–1192 (2002).
Zeng, X. et al. DNA polymerase eta is an A-T mutator in somatic hypermutation of immunoglobulin variable genes. Nature Immunol. 2, 537–541 (2001).
Zan, H. et al. The translesion DNA polymerase ζ plays a major role in Ig and bcl-6 somatic hypermutation. Immunity 14, 643–653 (2001).
Diaz, M., Verkoczy, L.K., Flajnik, M.F. & Klinman, N.R. Decreased frequency of somatic hypermutation and impaired affinity maturation but intact germinal center formation in mice expressing antisense RNA to DNA polymerase ζ. J. Immunol. 167, 327–335 (2001).
Diaz, M. & Casali, P. Somatic immunoglobulin hypermutation. Curr. Opin. Immunol. 14, 235–240 (2002).
Bachl, J. & Wabl, M. An immunoglobulin mutator that targets G. C base pairs. Proc. Natl. Acad. Sci. USA 93, 851–855 (1996).
Rada, C., Ehrenstein, M.R., Neuberger, M.S. & Milstein, C. Somatic hypermutation in MSH2-deficient mice is more focused on intrinsic hot spots suggesting hypermutation targeting to be a two stage process. Immunity 9, 135–141 (1998).
Weill, J.-C. et al. Ig gene hypermutation: a mechanism is due. Adv. Immunol. 80, 183–202 (2002).
Baba, T.W., Giroir, B.P. & Humphries, E.H. Cell lines derived from avian lymphomas exhibit two distinct phenotypes. Virology 144, 139–151 (1985).
Aoufouchi, S. et al. Two novel human and mouse DNA polymerases of the polX family. Nucleic Acids Res. 28, 3684–3693 (2000).
Aoufouchi, S. Monoclonal antibodies A4. 3.3; A6.4.12; B3.3.9; B15.4.13 anti-poly(ADP-ribose) polymerase. Hybridoma 16, 583 (1997).
Schlissel, M.S. Structure of nonhairpin coding-end DNA breaks in cells undergoing V(D)J recombination. Mol. Cell. Biol. 18, 2029–2037 (1998).
Mueller, P.R. & Wold, B. In vivo footprinting of a muscle specific enhancer by ligation mediated PCR. Science 246, 780–786 (1989).
Acknowledgements
We thank S. Lebecque for his advice on the BL2 cell line; B. Fleckenstein for the CB15 cell line; M. Braun for identifying the nonfunctional heavy chain allele of the BL2 cell line; A. Dahan for advice with the LM-PCR assay; A. Alcaïs for help with statistical analysis; and C. Hivroz for testing signal transduction pathways during the induction assay. Supported by the Fondation Princesse Grace de Monaco and the Ligue Nationale Française contre le Cancer (Equipe labellisée). A. F. and S. A. were supported during part of this work by the Fondation de France (Fondation contre la Leucémie).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Web Fig. 1.
Monitoring apoptosis, which occurs during induction of somatic hypermutation in the BL2 cell line through PARP proteolysis. Cells were induced to undergo V gene hypermutation (see Methods). At the indicated time points after induction, cell lysates were analyzed by immunoblotting with anti-PARP antibodies and HRP-coupled secondary antibodies. Appearance of the 86 kD cleavage product of the PARP protein monitors apoptosis occurring in BL2 cells. (JPG 5 kb)
Web Fig. 2.
Distribution of mutations obtained by the three antibodies stimulation procedure along the V4-39-JH5 gene of the BL2 cell line. The distribution of mutations listed in Table 2 is represented along the V4-39-JH5 sequence, with open triangles for deletions and closed triangles for insertions. The position of CDRs (complementarity-determining regions) is indicated. (JPG 60 kb)
Web Fig. 3.
Control of RNA transcription inhibition by actinomycin D during induction of somatic mutation in the BL2 cell line. Quantification of the unstable c-Myc transcripts versus actin transcripts was performed at the following time points: time 0, before incubation of BL2 with actinomycin D; 2 h, after 2 h incubation with actinomycin D at which time cells were induced for somatic mutation; 4 h 30, at the end of the induction procedure at which time mutations are analyzed (1 h at 4 °C with the antibody cocktail, followed by 90 min incubation at 37 °C). Mutation data are shown in Table 4. (JPG 4 kb)
Rights and permissions
About this article
Cite this article
Faili, A., Aoufouchi, S., Guéranger, Q. et al. AID-dependent somatic hypermutation occurs as a DNA single-strand event in the BL2 cell line. Nat Immunol 3, 815–821 (2002). https://doi.org/10.1038/ni826
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni826
This article is cited by
-
Multi-platform profiling characterizes molecular subgroups and resistance networks in chronic lymphocytic leukemia
Nature Communications (2021)
-
Lamin B1 regulates somatic mutations and progression of B-cell malignancies
Leukemia (2018)
-
Mutations, kataegis and translocations in B cells: understanding AID promiscuous activity
Nature Reviews Immunology (2016)
-
Chromatin remodeller SMARCA4 recruits topoisomerase 1 and suppresses transcription-associated genomic instability
Nature Communications (2016)
-
The chick chorioallantoic membrane as an in vivo xenograft model for Burkitt lymphoma
BMC Cancer (2014)