Abstract
Obesity is associated with metabolic inflammation and endoplasmic reticulum (ER) stress, both of which promote metabolic disease progression. Adipose tissue macrophages (ATMs) are key players orchestrating metabolic inflammation, and ER stress enhances macrophage activation. However, whether ER stress pathways underlie ATM regulation of energy homeostasis remains unclear. Here, we identified inositol-requiring enzyme 1α (IRE1α) as a critical switch governing M1–M2 macrophage polarization and energy balance. Myeloid-specific IRE1α abrogation in Ern1f/f; Lyz2-Cre mice largely reversed high-fat diet (HFD)-induced M1–M2 imbalance in white adipose tissue (WAT) and blocked HFD-induced obesity, insulin resistance, hyperlipidemia and hepatic steatosis. Brown adipose tissue (BAT) activity, WAT browning and energy expenditure were significantly higher in Ern1f/f; Lyz2-Cre mice. Furthermore, IRE1α ablation augmented M2 polarization of macrophages in a cell-autonomous manner. Thus, IRE1α senses protein unfolding and metabolic and immunological states, and consequently guides ATM polarization. The macrophage IRE1α pathway drives obesity and metabolic syndrome through impairing BAT activity and WAT browning.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Accession codes
References
Lumeng, C.N., Bodzin, J.L. & Saltiel, A.R. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J. Clin. Invest. 117, 175–184 (2007).
Hotamisligil, G.S. Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell 140, 900–917 (2010).
Lumeng, C.N. & Saltiel, A.R. Inflammatory links between obesity and metabolic disease. J. Clin. Invest. 121, 2111–2117 (2011).
Rosen, E.D. & Spiegelman, B.M. What we talk about when we talk about fat. Cell 156, 20–44 (2014).
Brestoff, J.R. & Artis, D. Immune regulation of metabolic homeostasis in health and disease. Cell 161, 146–160 (2015).
Biswas, S.K. & Mantovani, A. Orchestration of metabolism by macrophages. Cell Metab. 15, 432–437 (2012).
Odegaard, J.I. & Chawla, A. Alternative macrophage activation and metabolism. Annu. Rev. Pathol. 6, 275–297 (2011).
Osborn, O. & Olefsky, J.M. The cellular and signaling networks linking the immune system and metabolism in disease. Nat. Med. 18, 363–374 (2012).
McNelis, J.C. & Olefsky, J.M. Macrophages, immunity, and metabolic disease. Immunity 41, 36–48 (2014).
Chawla, A., Nguyen, K.D. & Goh, Y.P. Macrophage-mediated inflammation in metabolic disease. Nat. Rev. Immunol. 11, 738–749 (2011).
Odegaard, J.I. & Chawla, A. The immune system as a sensor of the metabolic state. Immunity 38, 644–654 (2013).
Gordon, S. Alternative activation of macrophages. Nat. Rev. Immunol. 3, 23–35 (2003).
Nguyen, K.D. et al. Alternatively activated macrophages produce catecholamines to sustain adaptive thermogenesis. Nature 480, 104–108 (2011).
Qiu, Y. et al. Eosinophils and type 2 cytokine signaling in macrophages orchestrate development of functional beige fat. Cell 157, 1292–1308 (2014).
Rao, R.R. et al. Meteorin-like is a hormone that regulates immune-adipose interactions to increase beige fat thermogenesis. Cell 157, 1279–1291 (2014).
Ron, D. & Walter, P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat. Rev. Mol. Cell Biol. 8, 519–529 (2007).
Walter, P. & Ron, D. The unfolded protein response: from stress pathway to homeostatic regulation. Science 334, 1081–1086 (2011).
Hetz, C. The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nat. Rev. Mol. Cell Biol. 13, 89–102 (2012).
Hetz, C., Martinon, F., Rodriguez, D. & Glimcher, L.H. The unfolded protein response: integrating stress signals through the stress sensor IRE1alpha. Physiol. Rev. 91, 1219–1243 (2011).
Boden, G. et al. Increase in endoplasmic reticulum stress-related proteins and genes in adipose tissue of obese, insulin-resistant individuals. Diabetes 57, 2438–2444 (2008).
Gregor, M.F. et al. Endoplasmic reticulum stress is reduced in tissues of obese subjects after weight loss. Diabetes 58, 693–700 (2009).
Maurel, M., Chevet, E., Tavernier, J. & Gerlo, S. Getting RIDD of RNA: IRE1 in cell fate regulation. Trends Biochem. Sci. 39, 245–254 (2014).
Shao, M. et al. Hepatic IRE1alpha regulates fasting-induced metabolic adaptive programs through the XBP1s-PPARα axis signalling. Nat. Commun. 5, 3528 (2014).
Kajimura, S., Spiegelman, B.M. & Seale, P. Brown and beige fat: physiological roles beyond heat generation. Cell Metab. 22, 546–559 (2015).
Jordan, M.B., van Rooijen, N., Izui, S., Kappler, J. & Marrack, P. Liposomal clodronate as a novel agent for treating autoimmune hemolytic anemia in a mouse model. Blood 101, 594–601 (2003).
Feng, B. et al. Clodronate liposomes improve metabolic profile and reduce visceral adipose macrophage content in diet-induced obese mice. PLoS One 6, e24358 (2011).
Murray, P.J. et al. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity 41, 14–20 (2014).
Mao, T. et al. PKA phosphorylation couples hepatic inositol-requiring enzyme 1alpha to glucagon signaling in glucose metabolism. Proc. Natl. Acad. Sci. USA 108, 15852–15857 (2011).
Wang, X.X. et al. Isolation of high quality RNA and construction of a suppression subtractive hybridization library from ramie (Boehmeria nivea L. Gaud.). Mol. Biol. Rep. 37, 2099–2103 (2010).
Martinon, F., Chen, X., Lee, A.H. & Glimcher, L.H. TLR activation of the transcription factor XBP1 regulates innate immune responses in macrophages. Nat. Immunol. 11, 411–418 (2010).
Kim, S. et al. Endoplasmic reticulum stress-induced IRE1alpha activation mediates cross-talk of GSK-3beta and XBP-1 to regulate inflammatory cytokine production. J. Immunol. 194, 4498–4506 (2015).
Shinoda, K. et al. Genetic and functional characterization of clonally derived adult human brown adipocytes. Nat. Med. 21, 389–394 (2015).
Wu, J. et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell 150, 366–376 (2012).
Satoh, T. et al. The Jmjd3-Irf4 axis regulates M2 macrophage polarization and host responses against helminth infection. Nat. Immunol. 11, 936–944 (2010).
Eguchi, J. et al. Interferon regulatory factor 4 regulates obesity-induced inflammation through regulation of adipose tissue macrophage polarization. Diabetes 62, 3394–3403 (2013).
Liao, X. et al. Kruppel-like factor 4 regulates macrophage polarization. J. Clin. Invest. 121, 2736–2749 (2011).
Odegaard, J.I. et al. Macrophage-specific PPARγ controls alternative activation and improves insulin resistance. Nature 447, 1116–1120 (2007).
Kang, K. et al. Adipocyte-derived Th2 cytokines and myeloid PPARdelta regulate macrophage polarization and insulin sensitivity. Cell Metab. 7, 485–495 (2008).
Volkmann, K. et al. Potent and selective inhibitors of the inositol-requiring enzyme 1 endoribonuclease. J. Biol. Chem. 286, 12743–12755 (2011).
Cross, B.C. et al. The molecular basis for selective inhibition of unconventional mRNA splicing by an IRE1-binding small molecule. Proc. Natl. Acad. Sci. USA 109, E869–E878 (2012).
Wang, M. & Kaufman, R.J. Protein misfolding in the endoplasmic reticulum as a conduit to human disease. Nature 529, 326–335 (2016).
Ozcan, U. et al. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science 306, 457–461 (2004).
Hui, X. et al. Adiponectin enhances cold-induced browning of subcutaneous adipose tissue via promoting M2 macrophage proliferation. Cell Metab. 22, 279–290 (2015).
Talukdar, S. et al. Neutrophils mediate insulin resistance in mice fed a high-fat diet through secreted elastase. Nat. Med. 18, 1407–1412 (2012).
Petruzzelli, M. et al. A switch from white to brown fat increases energy expenditure in cancer-associated cachexia. Cell Metab. 20, 433–447 (2014).
Mauer, J. et al. Signaling by IL-6 promotes alternative activation of macrophages to limit endotoxemia and obesity-associated resistance to insulin. Nat. Immunol. 15, 423–430 (2014).
Trapnell, C., Pachter, L. & Salzberg, S.L. TopHat: discovering splice junctions with RNA-Seq. Bioinformatics 25, 1105–1111 (2009).
Trapnell, C. et al. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat. Protoc. 7, 562–578 (2012).
Hong, F. et al. RankProd: a bioconductor package for detecting differentially expressed genes in meta-analysis. Bioinformatics 22, 2825–2827 (2006).
Yang, L. et al. A Phos-tag-based approach reveals the extent of physiological endoplasmic reticulum stress. PLoS One 5, e11621 (2010).
Acknowledgements
We thank S. Kajimura from UCSF for the beige preadipocytes and C. Jiang from Peking University for assistance with the macrophage depletion experiments. This work was supported by grants from the Ministry of Science and Technology (2016YFA0500100 and 973 Program 2012CB524900) and the National Natural Science Foundation of China (81420108006, 31690102 and 31230036) to Y.L.; the National Natural Science Foundation of China (31671181 and 31371153) to S.Z.D.; the National Natural Science Foundation of China (91539107) to Jianmiao Liu; and the National Natural Science Foundation of China (31671227 and 91642113) to Y.Q. This work was also supported by a research grant from the European Foundation for the Study of Diabetes/Chinese Diabetes Society/Lilly Programme to Y.L.
Author information
Authors and Affiliations
Contributions
B. Shan, S.-Z.D. and Y.L. conceived and designed the studies. B. Shan, X.W. and Y.W. performed most of the experiments and analyzed the data. C.X. and J.-D.J.H. conducted the bioinformatics analysis. Z.X., J.D., M.S., S.H., F.Z., L.Y. and M.Z. performed some of the animal and cell experiments. F.N., J. Li, Jianmiao Liu, Jianfeng Liu, Y.Q., W.J., B. Song, S.-Z.D. and L.R. provided essential reagents and assisted with experimental design and data analysis. B. Shan and Y.L. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Metabolic ER stress and inflammation in white adipose tissue (WAT) of mice with dietary obesity.
Male C57BL/6J mice were fed a normal chow (NC, 10% fat) or a high-fat diet (HFD, 60% fat) for 16 weeks, starting at 6 weeks of age (n=18 per group). (a) Body weight. (b) Body fat content. (c-f) Stromal vascular fraction (SVF) and adipocytes were prepared from epididymal (ep) WAT. Total RNA was extracted for quantitative RT-PCR analysis. (c,d) Xbp1 mRNA splicing and relative mRNA abundance of the indicated UPR marker genes in SVF (c) and adipocytes (d). (e,f) Relative mRNA abundance of the indicated proinflammatory genes in SVF (e) and adipocytes (f). (g,h) CD11b+ cells were isolated by magnetic MicroBeads from SVF of epWAT. Quantitative RT-PCR analysis of Xbp1 mRNA splicing and relative mRNA abundance of the indicated UPR marker and RIDD target genes (g) along with proinflammatory genes (h). Data are shown as mean ± s.e.m., *P < 0.05; **P < 0.01; ***P < 0.001 by Student’s t-test.
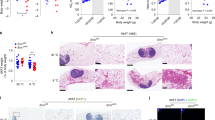
Supplementary Figure 2 Metabolic characterization of Ern1f/f; Lyz2-Cre mice.
(a) Immunoblot analyses of IRE1α protein in the indicated tissues of male Ern1f/f (f/f) and Ern1f/f; Lyz2-Cre (f/f:Cre) mice. (b-l) Male Ern1f/f; Lyz2-Cre mice and age-matched Ern1f/f littermates were fed an NC (n=8 per group) or HFD (n=10 per group) for 16 weeks, starting at 8 weeks of age. (b) Representative image of mice of the indicated genotype. (c) The tibia length of NC-fed mice. (d) Weight of epWAT from NC-fed mice. (e) Body weight gain of HFD-fed mice. (f) Representative images and weight of the indicated WAT from HFD-fed mice. (g) Lean mass was determined for mice fed an NC or HFD. (h) Immunoblot analyses of phosphorylation of AKT (p-AKT) at Ser473 in livers, muscle, and epWAT of HFD-fed mice after they were injected intravenously with PBS (-) or insulin (2 U/kg). (i) Liver weight was measured for NC- and HFD-fed mice. (j) Averaged daily food intake of NC- and HFD-fed mice. (k) Fecal TG content from HFD-fed mice. (l) Locomotor activity measured over a 24-h period. Results are shown as mean ± s.e.m., *P < 0.05; ***P < 0.001 by Student’s t-test or two-way ANOVA.
Supplementary Figure 3 Analysis of ATMs in NC-fed mice and ER stress in WAT of HFD-fed mice.
(a,b) SVFs of epWAT were prepared from NC-fed male Ern1f/f; Lyz2-Cre mice (n=4 per group) and their Ern1f/f littermates (n=3 per group) at 20 weeks of age. (a) Representative histograms of flow cytometry analysis of CD11b and F4/80 expression. Shown also are percentages of F4/80+CD11b+ cells. (b) Expression of CD206 in CD11b+ cells. Amounts of CD11b+CD206+ cells are quantified and shown as relative mean fluorescence intensity (MFI). (c-f) Ern1f/f; Lyz2-Cre mice (n=10 per group) and age-matched Ern1f/f littermates (n=8 per group) were fed an HFD for 16 weeks. Quantitative RT-PCR analysis of exon 2-containing Ern1 mRNA and Xbp1 mRNA splicing, along with the abundance of the indicated UPR genes in SVF (c,e) or adipocytes (d,f) from epWAT (c,d) and scWAT (e,f). Results are presented as mean ± s.e.m., *P < 0.05; **P<0.01; ***P<0.001 by Student’s t-test.
Supplementary Figure 4 Flow cytometry analysis of WAT macrophages and neutrophils.
SVFs were prepared from epWAT (a) and scWAT (b) of Ern1f/f or Ern1f/f; Lyz2-Cre mice following HFD feeding for 16 weeks (n=5 per group). CD11b+ cells were isolated with magnetic MicroBeads and then subjected to flow cytometry. Shown are representative histograms and percentages of CD45+CD11b+ cells, which were further analyzed for percentages of F4/80+CD11b+ macrophages and Ly6G+CD11b+ neutrophils.
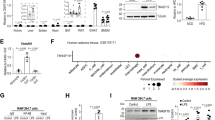
Supplementary Figure 5 Analysis of IRE1α phosphorylation and UPR activation in LPS- or IL-4-stimulated BMDMs.
BMDMs from mice of the indicated genotype were stimulated with 100 ng/ml LPS (a,b) or 20 ng/ml IL-4 (c,d) for the indicated times (3 and 4 independent experiments, respectively). (a,c) Phos-tag gel analysis of phosphorylation of IRE1α. BMDMs treated with thapsigargin (Tg, 1 μM) for 4 hours were used as a control. Shown are representative immunoblots with two different exposure times. (b,d) Quantification of eIF2a phosphorylation and BiP protein expression from the immunoblots in Fig. 6a and 6d, respectively. Results are presented as mean ± s.e.m.
Supplementary Figure 6 Effects of IRE1α abrogation on the IL-4-induced transcriptome in BMDMs.
BMDMs from Ern1f/f or Ern1f/f; Lyz2-Cre mice were treated with IL-4 for 24 hours. Total cellular RNAs were subjected to RNA-seq analysis (2 independent experiments). (a) Heat maps showing IL-4 induction of differentially expressed genes. Pie charts indicate the percentage of IL-4-upregulated or -downregulated genes which are further enhanced, attenuated or unaltered by IRE1α ablation in the presence of IL-4 stimulation (Ern1f/f; Lyz2-Cre_Ern1f/f). Gene expression results were analyzed by two-tailed rank product non-parametric method. (b) Gene Set Enrichment Analysis (GSEA) using the KEGG database (http://www.genome.jp/kegg/) with a nominal P-value < 0.05 and false discovery rate (FDR) < 0.25. Shown are heat maps for the indicated IL-4-enhanced or -suppressed cellular function processes and signaling pathways, as aligned with those affected by IRE1α deficiency under stimulation by IL-4 (Ern1f/f; Lyz2-Cre_Ern1f/f). (c) Changes in the expression of genes encoding potential secretory proteins as a result of IRE1α deficiency. Shown are heat maps for IL-4-upregulated or -downregulated genes, as aligned with those altered in IRE1α-deficient BMDMs relative to control cells under IL-4 stimulation (Ern1f/f; Lyz2-Cre_Ern1f/f).
Supplementary Figure 7 Enforced expression of XBP1s has no effect on the mRNA abundance of M2 polarization regulators.
Mouse BMDMs were infected by control or XBP1s-expressing lentiviruses before treatment with veh. or IL-4 for 24 hours (4 independent experiments). Quantitative RT-PCR analysis of the mRNA abundance of the XBP1s target gene Erdj4 (a) and the indicated regulators of M2 polarization (b). Data are shown as mean ± s.e.m., **P < 0.01 by two-way ANOVA.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–7 and Supplementary Table 1 (PDF 1419 kb)
Rights and permissions
About this article
Cite this article
Shan, B., Wang, X., Wu, Y. et al. The metabolic ER stress sensor IRE1α suppresses alternative activation of macrophages and impairs energy expenditure in obesity. Nat Immunol 18, 519–529 (2017). https://doi.org/10.1038/ni.3709
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.3709
This article is cited by
-
Endoplasmic reticulum stress and therapeutic strategies in metabolic, neurodegenerative diseases and cancer
Molecular Medicine (2024)
-
A nanoemulsion targeting adipose hypertrophy and hyperplasia shows anti-obesity efficiency in female mice
Nature Communications (2024)
-
The endoplasmic reticulum stress protein GRP94 modulates cathepsin L activity in M2 macrophages in conditions of obesity-associated inflammation and contributes to their pro-inflammatory profile
International Journal of Obesity (2024)
-
Glucocorticoids increase adiposity by stimulating Krüppel-like factor 9 expression in macrophages
Nature Communications (2024)
-
Endoplasmic Reticulum Stress in Hypertension and Salt Sensitivity of Blood Pressure
Current Hypertension Reports (2024)