Abstract
A20 is an anti-inflammatory protein linked to multiple human diseases; however, the mechanisms by which A20 prevents inflammatory disease are incompletely defined. We found that A20-deficient T cells and fibroblasts were susceptible to caspase-independent and kinase RIPK3–dependent necroptosis. Global deficiency in RIPK3 significantly restored the survival of A20-deficient mice. A20-deficient cells exhibited exaggerated formation of RIPK1-RIPK3 complexes. RIPK3 underwent physiological ubiquitination at Lys5 (K5), and this ubiquitination event supported the formation of RIPK1-RIPK3 complexes. Both the ubiquitination of RIPK3 and formation of the RIPK1-RIPK3 complex required the catalytic cysteine of A20's deubiquitinating motif. Our studies link A20 and the ubiquitination of RIPK3 to necroptotic cell death and suggest additional mechanisms by which A20 might prevent inflammatory disease.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Change history
21 May 2015
In the version of this article initially published, the filled circles in Figure 3b were incorrectly labeled 'A20+/flCD4-Cre'. The correct label is 'A20fl/flCD4-Cre'. The error has been corrected in the HTML and PDF versions of the article.
References
Opipari, A.W. Jr., Boguski, M.S. & Dixit, V.M. The A20 cDNA induced by tumor necrosis factor alpha encodes a novel type of zinc finger protein. J. Biol. Chem. 265, 14705–14708 (1990).
Lee, E.G. et al. Failure to regulate TNF-induced NF-κB and cell death responses in A20-deficient mice. Science 289, 2350–2354 (2000).
Wertz, I.E. et al. De-ubiquitination and ubiquitin ligase domains of A20 downregulate NF-κB signalling. Nature 430, 694–699 (2004).
Boone, D.L. et al. The ubiquitin modifiying enzyme A20 is essential for terminating TLR signaling. Nat. Immunol. 5, 1052–1060 (2004).
Ma, A. & Malynn, B.A. A20: linking ubiquitination with immunity and human diseases. Nat. Rev. Immunol. 12, 774–785 (2012).
Catrysse, L., Vereecke, L., Beyaert, R. & van Loo, G. A20 in inflammation and autoimmunity. Trends Immunol. 35, 22–31 (2014).
Musone, S. et al. Multiple polymorphisms in the TNFAIP3 region are independently associated with systemic lupus erythematosus. Nat. Genet. 40, 1062–1064 (2008).
Adrianto, I. et al. Association of a functional variant downstream of TNFAIP3 with systemic lupus erythematosus. Nat. Genet. 43, 253–258 (2011).
Turer, E.E. et al. Homeostatic MyD88-dependent signals cause lethal inflammation in the absence of A20. J. Exp. Med. 205, 451–464 (2008).
Hitotsumatsu, O. et al. The ubiquitin-editing enzyme A20 restricts nucleotide-binding oligomerization domain containing 2-triggered signals. Immunity 28, 381–390 (2008).
Kool, M. et al. The ubiquitin-editing protein A20 prevents dendritic cell activation, recognition of apoptotic cells, and systemic autoimmunity. Immunity 35, 82–96 (2011).
Hammer, G.E. et al. Expression of A20 by dendritic cells preserves immune homeostasis and prevents colitis and spondyloarthritis. Nat. Immunol. 12, 1184–1193 (2011).
Matmati, M. et al. A20 (TNFAIP3) deficiency in myeloid cells triggers erosive polyarthritis resembling rheumatoid arthritis. Nat. Genet. 43, 908–912 (2011).
Heger, K. et al. A20 deficient mast cells exacerbate inflammatory responses in vivo. PLoS Biol. 12, e1001762 (2014).
Tavares, R.M. et al. The ubiquitin modifying enzyme A20 restricts B cell survival and prevents autoimmunity. Immunity 33, 181–191 (2010).
Chu, Y. et al. B cells lacking the tumor suppressor TNFAIP3/A20 display impaired differentiation and hyperactivation and cause inflammation and autoimmunity in aged mice. Blood 117, 2227–2236 (2011).
Hövelmeyer, N. et al. A20 deficiency in B cells enhances B-cell proliferation and results in the development of autoantibodies. Eur. J. Immunol. 41, 595–601 (2011).
Chen, Z.J. & Sun, L. J Nonproteolytic functions of ubiquitin in cell signaling. Mol. Cell 33, 275–286 (2009).
Lu, T.T. et al. Dimerization and ubiquitin mediated recruitment of A20, a complex deubiquitinating enzyme. Immunity 38, 896–905 (2013).
Lin, S.C. et al. Molecular basis for the unique deubiquitinating activity of the NF-κB inhibitor A20. J. Mol. Biol. 376, 526–540 (2008).
Shembade, N., Ma, A. & Harhaj, E.W. Inhibition of NF-κB signaling by A20 through disruption of ubiquitin enzyme complexes. Science 327, 1135–1139 (2010).
Bosanac, I. et al. Ubiquitin binding to A20 ZnF4 is required for modulation of NF-κB signaling. Mol. Cell 40, 548–557 (2010).
Skaug, B. et al. Direct, noncatalytic mechanism of IKK inhibition by A20. Mol. Cell 44, 559–571 (2011).
Tokunaga, F. et al. Specific recognition of linear polyubiquitin by A20 zinc finger 7 is involved in NF-κB regulation. EMBO J. 31, 3856–3870 (2012).
Verhelst, K. et al. A20 inhibits LUBAC-mediated NF-kB activation by linear polyubiquitin chains via its zinc finger 7. EMBO J. 31, 3845–3855 (2012).
Beyaert, R., Heyninck, K. & Van Huffel, S. A20 and A20 binding proteins as cellular inhibitors of NF-κB dependent gene expression and apoptosis. Biochem. Pharmacol. 60, 1143–1151 (2000).
Ch′en, I.L., Tsau, J.S., Molkentin, J.D., Komatsu, M. & Hedrick, S.M. Mechanisms of necroptosis in T cells. J. Exp. Med. 208, 633–641 (2011).
Osborn, S.L. et al. Fas-associated death domain (FADD) is a negative regulator of T-cell receptor mediated necroptosis. Proc. Natl. Acad. Sci. USA 107, 13034–13039 (2010).
He, S. et al. Receptor interacting protein kinase 3 determines cellular necroptotic responses to TNFa. Cell 137, 1100–1111 (2009).
Cho, Y. et al. Phosphorylation-driven assembly of the RIPK1-RIPK3 complex regulates programmed necrosis and virus-induced inflammation. Cell 137, 1112–1123 (2009).
Li, J. et al. The RIP1/RIP3 necrosome forms a functional amyloid signaling complex required for programmed necrosis. Cell 150, 339–350 (2012).
Zamvil, S.S. & Steinman, L. The T lymphocyte in experimental allergic encephalomyelitis. Annu. Rev. Immunol. 8, 579–621 (1990).
Cua, D.J. et al. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature 421, 744–748 (2003).
Linkermann, A. & Green, D.R. Necroptosis. N. Engl. J. Med. 370, 455–465 (2014).
Udeshi, N.D., Mertins, P., Svinkina, T. & Carr, S.A. Large-scale identification of ubiquitination sites by mass spectrometry. Nat. Protoc. 8, 1950–1960 (2013).
Vanlangenakker, N. et al. TNF-induced necroptosis in L929 cells is tightly regulated by multiple TNFR1 complex I and II members. Cell Death Dis. 2, e230 (2011).
Duong, B. et al. A20 restricts ubiquitination of pro-interleukin-1β protein complexes and suppresses NLRP3 inflammasome activity. Immunity 42, 55–67 (2015).
Opipari, A.W., Hu, H.M., Yabkowitz, R. & Dixit, V.M. The A20 zinc finger protein protects cells from tumor necrosis factor cytotoxicity. J. Biol. Chem. 267, 12424–12427 (1992).
Welz, P.S. et al. FADD prevents RIPK3-mediated epithelial cell necrosis and chronic intestinal inflammation. Nature 477, 330–334 (2011).
Bates, J.S. et al. Meta-analysis and imputation identifies a 109 kb risk haplotype spanning TNFAIP3 associated with lupus nephritis and hematologic manifestations. Genes Immun. 10, 470–477 (2009).
Jiang, X. et al. Expression of tumor necrosis factor α-induced protein 3 mRNA in peripheral blood mononuclear cells negatively correlates with disease severity in psoriasis vulgaris. Clin. Vaccine Immunol. 19, 1938–1942 (2012).
Kelly, C. et al. Expression of the inflammatory regulator A20 correlates with lung function in patients with cystic fibrosis. J. Cyst. Fibros. 12, 411–415 (2013).
Kaiser, W.J. et al. RIPK3 mediates the embryonic lethality of caspase-8-deficient mice. Nature 471, 368–372 (2011).
Oberst, A. et al. Catalytic activity of the caspase-8-FLIPL complex inhibits RIPK3-dependent necrosis. Nature 471, 363–367 (2011).
Zhang, H. et al. Functional complementation between FADD and RIPK1 in embryos and lymphocytes. Nature 471, 373–376 (2011).
O'Donnell, M.A. et al. Caspase 8 inhibits programmed necrosis by processing CYLD. Nat. Cell Biol. 13, 1437–1442 (2011).
Moquin, D.M. et al. CYLD deubiquitinates RIP1 in the TNFα-induced necrosome to facilitate kinase activation and programmed necrosis. PLoS ONE 8, e76841 (2013).
Bertrand, M.J. et al. Cellular inhibitors of apoptosis cIAP1 and cIAP2 are required for innate immunity signaling by the pattern recognition receptors NOD1 and NOD2. Immunity 30, 789–801 (2009).
Bertrand, M.J. et al. cIAP1 and cIAP2 facilitate cancer cell survival by functioning as E3 ligases that promote RIPK1 ubiquitination. Mol. Cell 30, 689–700 (2008).
McComb, S. et al. cIAP1 and cIAP2 limit macrophage necroptosis by inhibiting Rip1 and Rip3 activation. Cell Death Differ. 19, 1791–1801 (2012).
Sun, L. et al. Mixed lineage kinase domain-like protein mediates necrosis signaling downstream of RIPK3 kinase. Cell 148, 213–227 (2012).
Xie, T. et al. Structural insights into RIPK3-mediated necroptotic signaling. Cell Rep. 5, 70–78 (2013).
Molnarfi, N. et al. MHC class II-dependent B cell APC function is required for induction of CNS autoimmunity independent of myelin-specific antibodies. J. Exp. Med. 210, 2921–2937 (2013).
Acknowledgements
We thank X. Wang for Ripk3−/− mice; J.C. Patarroyo and N. Molnarfi for assistance with EAE experiments; and S. Oakes for discussions. Mass spectrometry analysis was provided by the Bio-Organic Biomedical Mass Spectrometry Resource at the University of California, San Francisco, supported by funding from the Biomedical Technology Research Centers program of the National Institute of General Medical Sciences of the US National Institutes of Health (8P41GM103481) and the Howard Hughes Medical Institute. Supported by the US National Institutes of Health (DK071939 and DK095693 (A.M.), and AI073737 and NS063008 (S.S.Z.)), the Kenneth Rainin Foundation (A.M.), the National Multiple Sclerosis Society (RG 4768 and RG 5180 (S.S.Z.); and U.S.), the Guthy Jackson Charitable Foundation (S.S.Z.), the Maisin Foundation (S.S.Z.) and The Crohn's and Colitis Foundation of America (M.O. and S.O.).
Author information
Authors and Affiliations
Contributions
M.O. performed necroptosis experiments and assisted with the manuscript; S.O. performed most of the T cell studies and assisted with the manuscript; U.S.-T. designed and performed most of the EAE experiments; J.O.-P. designed and performed mass spectrometry studies; T.L. performed initial experiments of necroptosis in MEFs; R.T. and M.I.W. performed initial T cell studies; T.P. performed EAE experiments; B.D. provided mice and cell lines for necroptosis experiments; R.A. assisted with breeding and mouse experiments; A.A. and J.B. provided technical assistance; H.W. analyzed RIPK3 structure; A.B. supervised mass spectrometry studies; B.A.M. supervised T cell and necroptosis studies and helped write the manuscript; S.S.Z. supervised EAE experiments; and A.M. supervised the overall study and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
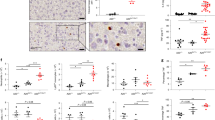
Supplementary Figure 1 Acute deletion of A20 from A20fl/flROSA26-ER-Cre T cells in vitro.
Quantitative real-time PCR analyses of A20 mRNA expression in purified CD4+ T cells from A20fl/fl ROSA-ER-Cre and A20+/fl ROSA-ER-Cre mice using the indicated doses of 4-OH-tamoxifen (4-OHT). Naïve CD4+ T cells were purified from the indicated mice, stimulated with agonist anti-CD3 and anti-CD28 antibodies and the indicated doses of 4-OHT for three days, and then stimulated with PMA/ionomycin for the indicated times (0, 1, or 3 h). Cells were harvested for qPCR analyses of A20 and Actin mRNA expression. Data represent ratios of A20/Actin mRNA expression. Note virtually complete deletion of A20 mRNA from A20fl/fl ROSA-ER-Cre cells at both doses of 4-OHT.
Supplementary Figure 2 Active Nec-1 is more effective than methylated (inactive) Nec-1 in rescuing A20-deficient T cells.
Naïve (CD62Lhi CD25lo) CD4+ T cells were FACS sorted from A20fl/fl CD4-Cre (CD45.1) and congenic A20+/fl CD4-Cre (CD45.2) mice, mixed 1:1 in co-cultures in vitro, and stimulated with anti-CD3 and anti-CD28 antibodies plus Z-VAD for the indicated time periods. Data reflect percentages of live cells after treatment with the RIPK1 kinase inhibitor, Nec-1 (10 μM), or an inactive (methylated) form of Nec-1 (10 μM). Data are displayed as a ratio of each genotype at each time point, normalized to the 1:1 ratio at the experiment’s initiation. Note that active Nec-1 selectively rescues A20fl/fl CD4-Cre T cells better than the inactive form of Nec-1.
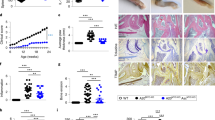
Supplementary Figure 3 A20 supports the differentiation of both TH1 and TH17 CD4+ T cells.
Naïve CD4+ T cells were FACS-purified from the indicated genotypes of mice, stimulated with agonist anti-CD3 and anti-CD28 antibodies in conjunction with either IL12 and anti-IL4 to polarize T cells toward TH1 cells or TGFβ, IL6, anti-IL4 and anti-IFNγ to polarize toward TH17 cells. Theumbers of CD4+ T cells expressing IFNγ or IL17A as well as the total numbers of live T cells were quantitated after 3 days. Note that the total numbers of A20fl/fl CD4-Cre T cells were markedly reduced under both TH1 and TH17 polarizing conditions compared to A20+/fl CD4-Cre T cells (as they are under neutral conditions, see Fig. 1d). Moreover, the specific numbers of TH1 (IFNγ+) T cells in TH1 polarizing cultures and the numbers of TH17 (IL17A+) T cells in TH17 polarizing conditions are also reduced in A20fl/fl CD4-Cre T cell cultures compared with A20+/fl CD4-Cre T cell cultures. Finally, the reduced numbers of total live CD4+ T cells, TH1 cells, and TH17 cells in A20fl/fl CD4-Cre T cell cultures are all significantly restored in A20fl/fl CD4-Cre Ripk3-/- T cell cultures. Increased T cell survival by RIPK3 deficiency occurs in A20fl/fl CD4-Cre T cells but not in A20+/fl CD4-Cre T cells, suggesting that A20fl/fl CD4-Cre T cells are selectively susceptible to RIPK3 dependent necroptosis.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–3 (PDF 106 kb)
Rights and permissions
About this article
Cite this article
Onizawa, M., Oshima, S., Schulze-Topphoff, U. et al. The ubiquitin-modifying enzyme A20 restricts ubiquitination of the kinase RIPK3 and protects cells from necroptosis. Nat Immunol 16, 618–627 (2015). https://doi.org/10.1038/ni.3172
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.3172
This article is cited by
-
Mediators of necroptosis: from cell death to metabolic regulation
EMBO Molecular Medicine (2024)
-
The Complexity of Being A20: From Biological Functions to Genetic Associations
Journal of Clinical Immunology (2024)
-
The double-edged functions of necroptosis
Cell Death & Disease (2023)
-
MLKL post-translational modifications: road signs to infection, inflammation and unknown destinations
Cell Death & Differentiation (2023)
-
RIPK3 promoter hypermethylation in hepatocytes protects from bile acid-induced inflammation and necroptosis
Cell Death & Disease (2023)