Abstract
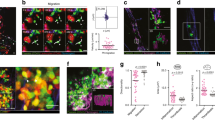
Through the use of intravital imaging of the liver, we demonstrate a collaborative role for platelets with Kupffer cells (KCs) in eradicating blood-borne bacterial infection. Under basal conditions, platelets, via the platelet-adhesion receptor GPIb, formed transient 'touch-and-go' interactions with von Willebrand factor (vWF) constitutively expressed on KCs. Bacteria such as Bacillus cereus and methicillin-resistant Staphylococcus aureus (MRSA) were rapidly caught by KCs and triggered platelets to switch from 'touch-and-go' adhesion to sustained GPIIb-mediated adhesion on the KC surface to encase the bacterium. Infected GPIbα-deficient mice had more endothelial and KC damage than did their wild-type counterparts, which led to more fluid leakage, substantial polycythemia and rapid mortality. Our study identifies a previously unknown surveillance mechanism by which platelets survey macrophages that rapidly converts to a critical host response to blood-borne bacteria.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Crispe, I.N. The liver as a lymphoid organ. Annu. Rev. Immunol. 27, 147–163 (2009).
Katz, S., Jimenez, M.A., Lehmkuhler, W.E. & Grosfeld, J.L. Liver bacterial clearance following hepatic artery ligation and portacaval shunt. J. Surg. Res. 51, 267–270 (1991).
Rabinovitch, M. Professional and non-professional phagocytes: an introduction. Trends Cell Biol. 5, 85–87 (1995).
Kobayashi, S.D. et al. Rapid neutrophil destruction following phagocytosis of Staphylococcus aureus. J. Innate Immun. 2, 560–575 (2010).
Pang, Y.Y. et al. agr-Dependent interactions of Staphylococcus aureus USA300 with human polymorphonuclear neutrophils. J. Innate Immun. 2, 546–559 (2010).
Dixon, T.C., Fadl, A.A., Koehler, T.M., Swanson, J.A. & Hanna, P.C. Early Bacillus anthracis-macrophage interactions: intracellular survival survival and escape. Cell Microbiol. 2, 453–463 (2000).
Guidi-Rontani, C., Levy, M., Ohayon, H. & Mock, M. Fate of germinated Bacillus anthracis spores in primary murine macrophages. Mol. Microbiol. 42, 931–938 (2001).
Guidi-Rontani, C., Weber-Levy, M., Labruyere, E. & Mock, M. Germination of Bacillus anthracis spores within alveolar macrophages. Mol. Microbiol. 31, 9–17 (1999).
Ramarao, N. & Lereclus, D. The InhA1 metalloprotease allows spores of the B. cereus group to escape macrophages. Cell Microbiol. 7, 1357–1364 (2005).
Mock, M. & Fouet, A. Anthrax. Annu. Rev. Microbiol. 55, 647–671 (2001).
Hoffmaster, A.R. et al. Identification of anthrax toxin genes in a Bacillus cereus associated with an illness resembling inhalation anthrax. Proc. Natl. Acad. Sci. USA 101, 8449–8454 (2004).
Miller, J.M. et al. Fulminating bacteremia and pneumonia due to Bacillus cereus. J. Clin. Microbiol. 35, 504–507 (1997).
Alfaro, D.V. III et al. Experimental posttraumatic Bacillus cereus endophthalmitis in a swine model. Efficacy of intravitreal ciprofloxacin, vancomycin, and imipenem. Retina 16, 317–323 (1996).
Guillemet, E. et al. The InhA metalloproteases of Bacillus cereus contribute concomitantly to virulence. J. Bacteriol. 192, 286–294 (2010).
Tran, S.L. et al. Haemolysin II is a Bacillus cereus virulence factor that induces apoptosis of macrophages. Cell Microbiol. 13, 92–108 (2011).
Semple, J.W., Italiano, J.E. Jr. & Freedman, J. Platelets and the immune continuum. Nat. Rev. Immunol. 11, 264–274 (2011).
Aslam, R. et al. Platelet Toll-like receptor expression modulates lipopolysaccharide-induced thrombocytopenia and tumor necrosis factor-alpha production in vivo. Blood 107, 637–641 (2006).
Clark, S.R. et al. Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat. Med. 13, 463–469 (2007).
Weyrich, A.S. et al. Signal-dependent translation of a regulatory protein, Bcl-3, in activated human platelets. Proc. Natl. Acad. Sci. USA 95, 5556–5561 (1998).
Weyrich, A.S. et al. Dipyridamole selectively inhibits inflammatory gene expression in platelet-monocyte aggregates. Circulation 111, 633–642 (2005).
McDonald, B., Urrutia, R., Yipp, B.G., Jenne, C.N. & Kubes, P. Intravascular neutrophil extracellular traps capture bacteria from the bloodstream during sepsis. Cell Host Microbe 12, 324–333 (2012).
Yeaman, M.R., Bayer, A.S., Koo, S.P., Foss, W. & Sullam, P.M. Platelet microbicidal proteins and neutrophil defensin disrupt the Staphylococcus aureus cytoplasmic membrane by distinct mechanisms of action. J. Clin. Invest. 101, 178–187 (1998).
Yeaman, M.R., Tang, Y.Q., Shen, A.J., Bayer, A.S. & Selsted, M.E. Purification and in vitro activities of rabbit platelet microbicidal proteins. Infect. Immun. 65, 1023–1031 (1997).
Cole, A.M. et al. Cutting edge: IFN-inducible ELR-CXC chemokines display defensin-like antimicrobial activity. J. Immunol. 167, 623–627 (2001).
Kerrigan, S.W. et al. A role for glycoprotein Ib in Streptococcus sanguis-induced platelet aggregation. Blood 100, 509–516 (2002).
Kastrup, C.J. et al. Spatial localization of bacteria controls coagulation of human blood by 'quorum acting'. Nat. Chem. Biol. 4, 742–750 (2008).
Jenne, C.N., Wong, C.H., Petri, B. & Kubes, P. The use of spinning-disk confocal microscopy for the intravital analysis of platelet dynamics in response to systemic and local inflammation. PLoS ONE 6, e25109 (2011).
Yip, J., Shen, Y., Berndt, M.C. & Andrews, R.K. Primary platelet adhesion receptors. IUBMB Life 57, 103–108 (2005).
Fuchs, B. et al. Flow-based measurements of von Willebrand factor (VWF) function: binding to collagen and platelet adhesion under physiological shear rate. Thromb. Res. 125, 239–245 (2010).
Muta, T. & Iwanaga, S. The role of hemolymph coagulation in innate immunity. Curr. Opin. Immunol. 8, 41–47 (1996).
Yeaman, M.R. Platelets in defense against bacterial pathogens. Cell Mol. Life Sci. 67, 525–544 (2010).
Czapiga, M., Gao, J.L., Kirk, A. & Lekstrom-Himes, J. Human platelets exhibit chemotaxis using functional N-formyl peptide receptors. Exp. Hematol. 33, 73–84 (2005).
Yeaman, M.R. The role of platelets in antimicrobial host defense. Clin. Infect. Dis. 25, 951–968 (1997).
McMorran, B.J. et al. Platelets kill intraerythrocytic malarial parasites and mediate survival to infection. Science 323, 797–800 (2009).
Verschoor, A. et al. A platelet-mediated system for shuttling blood-borne bacteria to CD8α+ dendritic cells depends on glycoprotein GPIb and complement C3. Nat. Immunol. 12, 1194–1201 (2011).
Hoffmeister, K.M. et al. The clearance mechanism of chilled blood platelets. Cell 112, 87–97 (2003).
Garcia, V.V., Coppola, R. & Mannucci, P.M. The role of the spleen in regulating the plasma levels of factor VIII–von Willebrand's factor after DDAVP. Blood 60, 1402–1406 (1982).
Popova, T.G., Millis, B., Bailey, C. & Popov, S.G. Platelets, inflammatory cells, von Willebrand factor, syndecan-1, fibrin, fibronectin, and bacteria co-localize in the liver thrombi of Bacillus anthracis-infected mice. Microb. Pathog. 52, 1–9 (2011).
Altamura, M. et al. Splenectomy and sepsis: the role of the spleen in the immune-mediated bacterial clearance. Immunopharmacol. Immunotoxicol. 23, 153–161 (2001).
Wellmer, A. et al. Experimental pneumococcal meningitis: impaired clearance of bacteria from the blood due to increased apoptosis in the spleen in Bcl-2-deficient mice. Infect. Immun. 72, 3113–3119 (2004).
Lee, W.Y. et al. An intravascular immune response to Borrelia burgdorferi involves Kupffer cells and iNKT cells. Nat. Immunol. 11, 295–302 (2010).
Soong, L. et al. Disruption of CD40–CD40 ligand interactions results in an enhanced susceptibility to Leishmania amazonensis infection. Immunity 4, 263–273 (1996).
Van Rooijen, N. & Sanders, A. Kupffer cell depletion by liposome-delivered drugs: comparative activity of intracellular clodronate, propamidine, and ethylenediaminetetraacetic acid. Hepatology 23, 1239–1243 (1996).
van Rooijen, N., Sanders, A. & van den Berg, T.K. Apoptosis of macrophages induced by liposome-mediated intracellular delivery of clodronate and propamidine. J. Immunol. Methods 193, 93–99 (1996).
Dunn, A.K. & Handelsman, J. A vector for promoter trapping in Bacillus cereus. Gene 226, 297–305 (1999).
Wong, C.H., Jenne, C.N., Lee, W.Y., Leger, C. & Kubes, P. Functional innervation of hepatic iNKT cells is immunosuppressive following stroke. Science 334, 101–105 (2011).
Liu, L. et al. LSP1 is an endothelial gatekeeper of leukocyte transendothelial migration. J. Exp. Med. 201, 409–418 (2005).
Acknowledgements
We thank T. Chavakis (Dresden University of Technology) for Gp1ba−/− mice; K. McNagny (University of British Columbia) for GPIIb-deficient mice; J. Handelsman (Yale University) for making the green fluorescent protein–expressing B. cereus strain, and R.R. Pompano (University of Chicago) and R.F. Ismagilov (California Institute of Technology) for providing that strain; The Live Cell Imaging Facility funded by the Canadian Foundation for Innovation; and P. Colarusso for training and assistance related to microscopy. Supported by Alberta Innovates Health Solutions (C.H.Y.W., C.N.J. and P.K.), the Canadian Institutes of Health Research (P.K.) and the Canada Research Chairs Program (P.K.).
Author information
Authors and Affiliations
Contributions
C.H.Y.W. and C.N.J. designed and did most of the experiments; C.H.Y.W. prepared the manuscript; B.P. did all of the muscle, skin and ear experiments; N.L.C. contributed some liver-imaging experiments; C.N.J. made all of the manuscript revisions and did additional experiments; and P.K. provided overall supervision, helped design all of the experiments and prepared the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–4 (PDF 353 kb)
Supplementary Video 1
Platelet form "touch-and-go" interactions within liver sinusoids in vivo. Intravital visualization of circulating platelets within the liver microvasculature of a non-infected wild-type mouse using spinning-disk confocal fluorescence microscopy. Platelets were labeled with PE-conjugated anti-CD49b (red). Kupffer cells were labelled with Alexa Fluor 647-conjugated anti-F4/80. Elapsed time is shown at the top right. The time lapse was recorded at maximum speed and exported to video at 5 frames per second (fps). Objective: UPLANSAPO 20x/0.70. Scale bar, 50 μm. The video is representative of 2 independent experiments of minimum of 3 mice per experiment. (MOV 153 kb)
Supplementary Video 2
Platelet interactions and aggregation within the liver of a B. cereus infected mouse. Intravital visualization of circulating platelets, adhering and forming firm aggregates within the liver microvasculature during the first 10 min of GFP-expressing B. cereus infection using spinning-disk confocal fluorescence microscopy. Platelets were labeled with PE-conjugated anti-CD49b (red). Elapsed time is shown at the top right. The time lapse was recorded at maximum speed and exported to video at 20 frames per second (fps). Objective: UPLANSAPO 10x/0.40. Scale bar, 100 μm. The video is representative of 2 independent experiments of minimum of 3 mice per experiment. (MOV 474 kb)
Supplementary Video 3
Significant reduction of captured bacterium in KC-depleted animal. Intravital visualization of intravenous GFP-expressing B. cereus infection within the liver microvasculature of KC-depleted mouse using spinning-disk confocal fluorescence microscopy. Platelets were labeled with PE-conjugated anti-CD49b (red). Elapsed time is shown at the top right. The time lapse was recorded at maximum speed and exported to video at 5 frames per second (fps). Objective: UPLANSAPO 10x/0.40. Scale bar, 100 μm. The video is representative of 2 independent experiments of minimum of 3 mice per experiment. (MOV 1608 kb)
Rights and permissions
About this article
Cite this article
Wong, C., Jenne, C., Petri, B. et al. Nucleation of platelets with blood-borne pathogens on Kupffer cells precedes other innate immunity and contributes to bacterial clearance. Nat Immunol 14, 785–792 (2013). https://doi.org/10.1038/ni.2631
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.2631
This article is cited by
-
Limited value of platelet-related markers in diagnosing periprosthetic joint infection
BMC Musculoskeletal Disorders (2024)
-
EGFR of platelet regulates macrophage activation and bacterial phagocytosis function
Journal of Inflammation (2024)
-
Prognostic significance of early platelet dynamics in Staphylococcus aureus bacteremia
BMC Infectious Diseases (2023)
-
Assistive diagnostic indicators for infections related to lumbar posterior interbody fusion internal fixation: platelet count and mean platelet volume
Journal of Orthopaedic Surgery and Research (2023)
-
The Function and Regulation of Platelet P2Y12 Receptor
Cardiovascular Drugs and Therapy (2023)