Abstract
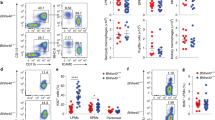
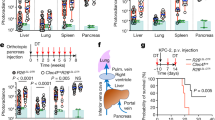
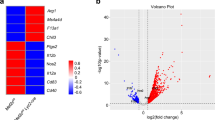
Macrophages are professional phagocytic cells that orchestrate innate immune responses and have considerable phenotypic diversity at different anatomical locations. However, the mechanisms that control the heterogeneity of tissue macrophages are not well characterized. Here we found that the nuclear receptor LXRα was essential for the differentiation of macrophages in the marginal zone (MZ) of the spleen. LXR-deficient mice were defective in the generation of MZ and metallophilic macrophages, which resulted in abnormal responses to blood-borne antigens. Myeloid-specific expression of LXRα or adoptive transfer of wild-type monocytes restored the MZ microenvironment in LXRα-deficient mice. Our results demonstrate that signaling via LXRα in myeloid cells is crucial for the generation of splenic MZ macrophages and identify an unprecedented role for a nuclear receptor in the generation of specialized macrophage subsets.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout









Similar content being viewed by others
References
Taylor, P.R. et al. Macrophage receptors and immune recognition. Annu. Rev. Immunol. 23, 901–944 (2005).
Medzhitov, R. Origin and physiological roles of inflammation. Nature 454, 428–435 (2008).
Elliott, M.R. & Ravichandran, K.S. Clearance of apoptotic cells: implications in health and disease. J. Cell Biol. 189, 1059–1070 (2010).
Gordon, S. & Taylor, P.R. Monocyte and macrophage heterogeneity. Nat. Rev. Immunol. 5, 953–964 (2005).
Hashimoto, D., Miller, J. & Merad, M. Dendritic cell and macrophage heterogeneity in vivo. Immunity 35, 323–335 (2011).
Geissmann, F. et al. Development of monocytes, macrophages, and dendritic cells. Science 327, 656–661 (2010).
Mebius, R.E. & Kraal, G. Structure and function of the spleen. Nat. Rev. Immunol. 5, 606–616 (2005).
Kraal, G. & Mebius, R. New insights into the cell biology of the marginal zone of the spleen. Int. Rev. Cytol. 250, 175–215 (2006).
den Haan, J.M. & Kraal, G. Innate immune functions of macrophage subpopulations in the spleen. J. Innate Immun. 4, 437–445 (2012).
Martin, F. & Kearney, J.F. Marginal-zone B cells. Nat. Rev. Immunol. 2, 323–335 (2002).
Repa, J.J. & Mangelsdorf, D.J. The role of orphan nuclear receptors in the regulation of cholesterol homeostasis. Annu. Rev. Cell Dev. Biol. 16, 459–481 (2000).
A-González, N. & Castrillo, A. Liver X receptors as regulators of macrophage inflammatory and metabolic pathways. Biochim. Biophys. Acta 1812, 982–994 (2011).
Glass, C.K. & Saijo, K. Nuclear receptor transrepression pathways that regulate inflammation in macrophages and T cells. Nat. Rev. Immunol. 10, 365–376 (2010).
Joseph, S.B., Castrillo, A., Laffitte, B.A., Mangelsdorf, D.J. & Tontonoz, P. Reciprocal regulation of inflammation and lipid metabolism by liver X receptors. Nat. Med. 9, 213–219 (2003).
A-González, N. et al. Apoptotic cells promote their own clearance and immune tolerance through activation of the nuclear receptor LXR. Immunity 31, 245–258 (2009).
Hong, C. et al. Coordinate regulation of neutrophil homeostasis by liver X receptors in mice. J. Clin. Invest. 122, 337–347 (2012).
Calkin, A.C. & Tontonoz, P. Transcriptional integration of metabolism by the nuclear sterol-activated receptors LXR and FXR. Nat. Rev. Mol. Cell Biol. 13, 213–224 (2012).
Streeter, P.R., Berg, E.L., Rouse, B.T., Bargatze, R.F. & Butcher, E.C. A tissue-specific endothelial cell molecule involved in lymphocyte homing. Nature 331, 41–46 (1988).
Garin, A. et al. Toll-like receptor 4 signaling by follicular dendritic cells is pivotal for germinal center onset and affinity maturation. Immunity 33, 84–95 (2010).
Hemmi, H. et al. A new triggering receptor expressed on myeloid cells (Trem) family member, Trem-like 4, binds to dead cells and is a DNAX activation protein 12-linked marker for subsets of mouse macrophages and dendritic cells. J. Immunol. 182, 1278–1286 (2009).
Nolte, M.A. et al. B cells are crucial for both development and maintenance of the splenic marginal zone. J. Immunol. 172, 3620–3627 (2004).
Karlsson, M.C. et al. Macrophages control the retention and trafficking of B lymphocytes in the splenic marginal zone. J. Exp. Med. 198, 333–340 (2003).
Lu, T.T. & Cyster, J.G. Integrin-mediated long-term B cell retention in the splenic marginal zone. Science 297, 409–412 (2002).
de Boer, J. et al. Transgenic mice with hematopoietic and lymphoid specific expression of Cre. Eur. J. Immunol. 33, 314–325 (2003).
Joseph, S.B. et al. LXR-dependent gene expression is important for macrophage survival and the innate immune response. Cell 119, 299–309 (2004).
Watanabe, Y. et al. Expression of the LXRalpha protein in human atherosclerotic lesions. Arterioscler. Thromb. Vasc. Biol. 25, 622–627 (2005).
He, W. et al. Development of a synthetic promoter for macrophage gene therapy. Hum. Gene Ther. 17, 949–959 (2006).
Li, G. et al. Macrophage LXRα gene therapy ameliorates atherosclerosis as well as hypertriglyceridemia in LDLR−/− mice. Gene Ther. 18, 835–841 (2011).
Varol, C. et al. Monocytes give rise to mucosal, but not splenic, conventional dendritic cells. J. Exp. Med. 204, 171–180 (2007).
Yona, S. et al. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under Homeostasis. Immunity 38, 79–91 (2013).
Hong, C. et al. Constitutive activation of LXR in macrophages regulates metabolic and inflammatory gene expression: identification of ARL7 as a direct target. J. Lipid Res. 52, 531–539 (2010).
Ingersoll, M.A. et al. Comparison of gene expression profiles between human and mouse monocyte subsets. Blood 115, e10–e19 (2010).
van Rooijen, N., Kors, N. & Kraal, G. Macrophage subset repopulation in the spleen: differential kinetics after liposome-mediated elimination. J. Leukoc. Biol. 45, 97–104 (1989).
Korf, H. et al. Liver X receptors contribute to the protective immune response against Mycobacterium tuberculosis in mice. J. Clin. Invest. 119, 1626–1637 (2009).
van Furth, R. & Cohn, Z.A. The origin and kinetics of mononuclear phagocytes. J. Exp. Med. 128, 415–435 (1968).
Ginhoux, F. et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science 330, 841–845 (2010).
Fogg, D.K. et al. A clonogenic bone marrow progenitor specific for macrophages and dendritic cells. Science 311, 83–87 (2006).
Hoeffel, G. et al. Adult Langerhans cells derive predominantly from embryonic fetal liver monocytes with a minor contribution of yolk sac-derived macrophages. J. Exp. Med. 209, 1167–1181 (2012).
Schulz, C. et al. A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science 336, 86–90 (2012).
Jenkins, S.J. et al. Local macrophage proliferation, rather than recruitment from the blood, is a signature of TH2 inflammation. Science 332, 1284–1288 (2011).
van Furth, R. Diesselhoff-den Dulk, M.M. Dual origin of mouse spleen macrophages. J. Exp. Med. 160, 1273–1283 (1984).
Kohyama, M. et al. Role for Spi-C in the development of red pulp macrophages and splenic iron homeostasis. Nature 457, 318–321 (2009).
Swirski, F.K. The spatial and developmental relationships in the macrophage family. Arterioscler. Thromb. Vasc. Biol. 31, 1517–1522 (2011).
Swirski, F.K. et al. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science 325, 612–616 (2009).
Hannedouche, S. et al. Oxysterols direct immune cell migration via EBI2. Nature 475, 524–527 (2011).
Bensinger, S.J. et al. LXR signaling couples sterol metabolism to proliferation in the acquired immune response. Cell 134, 97–111 (2008).
Villablanca, E.J. et al. Tumor-mediated liver X receptor-α activation inhibits CC chemokine receptor-7 expression on dendritic cells and dampens antitumor responses. Nat. Med. 16, 98–105 (2009).
Peet, D.J. et al. Cholesterol and bile acid metabolism are impaired in mice lacking the nuclear oxysterol receptor LXRα. Cell 93, 693–704 (1998).
Acknowledgements
We thank D. Mangelsdorf (University of Texas Southwestern Medical Center) for LXRα- and LXRβ-sufficient wild-type (Nr1h3+/+Nr1h2+/+) mice, LXRα-deficient (Nr1h3−/−) mice, LXRβ-deficient (Nr1h2−/−) mice and LXR-deficient (Nr1h3−/−Nr1h2−/−) mice; the Institut Clinique de la Souris for Nr1h3fl/fl mice; D. Kioussis (Medical Research Council) and S. Gonzalez (Centro Nacional de Investigaciones Cardiovasculares) for Vav-Cre mice; S. Gordon and M. Stacey (University of Oxford) for antibody to F4/80 (anti-F4/80) and anti-CD169; G. Randolph (Washington University St. Louis) for discussions and anti-TREML4 from the R. Steinman laboratory (Rockefeller University); M. Kosco-Vilbois (NovImmune) for anti-FDC-M1 and anti-FDC-M2; J. Collins and T. Willson (GlaxoSmithKline) for the synthetic ligands of LXR (GW3965) and retinoid X receptor (LG268); N. Ruddle, C. Glass, N. Spann, A. Chawla, G. Lemke, D. Hume, L. Hedrick, A. Lazarus and L. Bosca for comments; and Servicio Microscopia Electronica (University of Las Palmas de Gran Canaria) for electron microscopy. Supported by the Spanish Ministry of Research and Innovation (SAF2008-00057 to A.C.), the Ministry of Economy and Competitiveness (SAF2011-29244 to A.C. and SAF2009-11037 to A.H.), Framework Programme 7 of the European Union (International Reintegration Grant IRG246655 to A.H.), the Howard Hughes Medical Institute (P.T.), the US National Institutes of Health (HL-066088 and HL-030568 to P.T.), Subprograma Ramón y Cajal (RYC-2007-00697 to A.H.), Formación de Personal Investigador (BES-2010-032828 to M.C.-A. and BES-2009-012191 to I.H.H.) and Universidad Las Palmas de Gran Canaria (J.V.d.l.R.).
Author information
Authors and Affiliations
Contributions
N.A.-G., J.A.G., G.G. and M.D. designed and did experiments and analyzed data; J.V.d.l.R., I.H.H., M.C.-A., F.L., C.T. and S.B. did experiments; C.H., P.C.L., M.A., S.A., T.M., S.L., A.L.C., P.T. and A.H. provided reagents and intellectual input and analyzed or interpreted data; N.A.-G. and A.H. contributed to the writing of the manuscript; and A.C. supervised the project, designed and did experiments, analyzed data and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–9 and Supplementary Table 1 (PDF 1626 kb)
Rights and permissions
About this article
Cite this article
A-Gonzalez, N., Guillen, J., Gallardo, G. et al. The nuclear receptor LXRα controls the functional specialization of splenic macrophages. Nat Immunol 14, 831–839 (2013). https://doi.org/10.1038/ni.2622
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.2622
This article is cited by
-
Spleen regeneration after subcutaneous heterotopic autotransplantation in a mouse model
Biological Research (2023)
-
Inhibition of LXR controls the polarization of human inflammatory macrophages through upregulation of MAFB
Cellular and Molecular Life Sciences (2023)
-
Metabolism of tissue macrophages in homeostasis and pathology
Cellular & Molecular Immunology (2022)
-
A detailed analysis of innate and adaptive immune responsiveness upon infection with Salmonella enterica serotype Enteritidis in young broiler chickens
Veterinary Research (2021)
-
Targeting macrophages in cancer immunotherapy
Signal Transduction and Targeted Therapy (2021)