Abstract
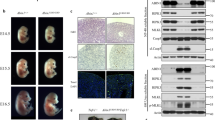
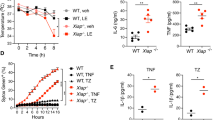
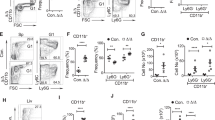
The E3 ligase ARIH2 has an unusual structure and mechanism of elongating ubiquitin chains. To understand its physiological role, we generated gene-targeted mice deficient in ARIH2. ARIH2 deficiency resulted in the embryonic death of C57BL/6 mice. On a mixed genetic background, the lethality was attenuated, with some mice surviving beyond weaning and then succumbing to an aggressive multiorgan inflammatory response. We found that in dendritic cells (DCs), ARIH2 caused degradation of the inhibitor IκBβ in the nucleus, which abrogated its ability to sequester, protect and transcriptionally coactivate the transcription factor subunit p65 in the nucleus. Loss of ARIH2 caused dysregulated activation of the transcription factor NF-κB in DCs, which led to lethal activation of the immune system in ARIH2-sufficent mice reconstituted with ARIH2-deficient hematopoietic stem cells. Our data have therapeutic implications for targeting ARIH2 function.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Marín, I., Lucas, J.I., Gradilla, A.C. & Ferrus, A. Parkin and relatives: the RBR family of ubiquitin ligases. Physiol. Genomics 17, 253–263 (2004).
van der Reijden, B.A., Erpelinck-Verschueren, C.A., Lowenberg, B. & Jansen, J.H. TRIADs: a new class of proteins with a novel cysteine-rich signature. Protein Sci. 8, 1557–1561 (1999).
Tan, N.G. et al. Characterisation of the human and mouse orthologues of the Drosophila ariadne gene. Cytogenet. Cell Genet. 90, 242–245 (2000).
Aguilera, M., Oliveros, M., Martinez-Padron, M., Barbas, J.A. & Ferrus, A. Ariadne-1: a vital Drosophila gene is required in development and defines a new conserved family of ring-finger proteins. Genetics 155, 1231–1244 (2000).
Wenzel, D.M., Lissounov, A., Brzovic, P.S. & Klevit, R.E. UBCH7 reactivity profile reveals parkin and HHARI to be RING/HECT hybrids. Nature 474, 105–108 (2011).
Chuang, T.H. & Ulevitch, R.J. Triad3A, an E3 ubiquitin-protein ligase regulating Toll-like receptors. Nat. Immunol. 5, 495–502 (2004).
Fearns, C., Pan, Q., Mathison, J.C. & Chuang, T.H. Triad3A regulates ubiquitination and proteasomal degradation of RIP1 following disruption of Hsp90 binding. J. Biol. Chem. 281, 34592–34600 (2006).
Nakhaei, P. et al. The E3 ubiquitin ligase Triad3A negatively regulates the RIG-I/MAVS signaling pathway by targeting TRAF3 for degradation. PLoS Pathog. 5, e1000650 (2009).
Ghosh, S. & Hayden, M.S. New regulators of NF-κB in inflammation. Nat. Rev. Immunol. 8, 837–848 (2008).
Vallabhapurapu, S. & Karin, M. Regulation and function of NF-κB transcription factors in the immune system. Annu. Rev. Immunol. 27, 693–733 (2009).
Mladek, C., Guger, K. & Hauser, M.T. Identification and characterization of the ARIADNE gene family in Arabidopsis. A group of putative E3 ligases. Plant Physiol. 131, 27–40 (2003).
Marteijn, J.A. et al. The ubiquitin ligase Triad1 inhibits myelopoiesis through UbcH7 and Ubc13 interacting domains. Leukemia 23, 1480–1489 (2009).
Wang, H., Bei, L., Shah, C.A., Horvath, E. & Eklund, E.A. HoxA10 influences protein ubiquitination by activating transcription of ARIH2, the gene encoding Triad1. J. Biol. Chem. 286, 16832–16845 (2011).
Pietschmann, K. et al. Differential regulation of PML-RAR α stability by the ubiquitin ligases SIAH1/SIAH2 and TRIAD1. Int. J. Biochem. Cell Biol. 44, 132–138 (2012).
Lee, E.G. et al. Failure to regulate TNF-induced NF-κB and cell death responses in A20-deficient mice. Science 289, 2350–2354 (2000).
Starr, R. et al. Liver degeneration and lymphoid deficiencies in mice lacking suppressor of cytokine signaling-1. Proc. Natl. Acad. Sci. USA 95, 14395–14399 (1998).
Yeh, W.C. et al. Early lethality, functional NF-κB activation, and increased sensitivity to TNF-induced cell death in TRAF2-deficient mice. Immunity 7, 715–725 (1997).
Chiffoleau, E. et al. TNF receptor-associated factor 6 deficiency during hemopoiesis induces Th2-polarized inflammatory disease. J. Immunol. 171, 5751–5759 (2003).
Turer, E.E. et al. Homeostatic MyD88-dependent signals cause lethal inflamMation in the absence of A20. J. Exp. Med. 205, 451–464 (2008).
Nguyen, L.T. et al. TRAF2 deficiency results in hyperactivity of certain TNFR1 signals and impairment of CD40-mediated responses. Immunity 11, 379–389 (1999).
Metcalf, D., Di Rago, L., Mifsud, S., Hartley, L. & Alexander, W.S. The development of fatal myocarditis and polymyositis in mice heterozygous for IFN-γ and lacking the SOCS-1 gene. Proc. Natl. Acad. Sci. USA 97, 9174–9179 (2000).
Alexander, W.S. et al. SOCS1 is a critical inhibitor of interferon γ signaling and prevents the potentially fatal neonatal actions of this cytokine. Cell 98, 597–608 (1999).
Asseman, C., Mauze, S., Leach, M.W., Coffman, R.L. & Powrie, F. An essential role for interleukin 10 in the function of regulatory T cells that inhibit intestinal inflammation. J. Exp. Med. 190, 995–1004 (1999).
Lutz, M.B. et al. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J. Immunol. Methods 223, 77–92 (1999).
Naik, S.H. et al. Development of plasmacytoid and conventional dendritic cell subtypes from single precursor cells derived in vitro and in vivo. Nat. Immunol. 8, 1217–1226 (2007).
Ohashi, P.S. et al. Ablation of “tolerance” and induction of diabetes by virus infection in viral antigen transgenic mice. Cell 65, 305–317 (1991).
Oldstone, M.B., Nerenberg, M., Southern, P., Price, J. & Lewicki, H. Virus infection triggers insulin-dependent diabetes mellitus in a transgenic model: role of anti-self (virus) immune response. Cell 65, 319–331 (1991).
Dissanayake, D. et al. Nuclear factor-κB1 controls the functional maturation of dendritic cells and prevents the activation of autoreactive T cells. Nat. Med. 17, 1663–1667 (2011).
Lin, A.C., Dissanayake, D., Dhanji, S., Elford, A.R. & Ohashi, P.S. Different toll-like receptor stimuli have a profound impact on cytokines required to break tolerance and induce autoimmunity. PLoS ONE 6, e23940 (2011).
Garza, K.M. et al. Role of antigen-presenting cells in mediating tolerance and autoimmunity. J. Exp. Med. 191, 2021–2027 (2000).
Suyang, H., Phillips, R., Douglas, I. & Ghosh, S. Role of unphosphorylated, newly synthesized IκBβ in persistent activation of NF-κB. Mol. Cell. Biol. 16, 5444–5449 (1996).
Rao, P. et al. IκBβ acts to inhibit and activate gene expression during the inflammatory response. Nature 466, 1115–1119 (2010).
Scheibel, M. et al. IκBβ is an essential co-activator for LPS-induced IL-1β transcription in vivo. J. Exp. Med. 207, 2621–2630 (2010).
McKinsey, T.A., Chu, Z.L. & Ballard, D.W. Phosphorylation of the PEST domain of IκBβ regulates the function of NF-κB/IκBβ complexes. J. Biol. Chem. 272, 22377–22380 (1997).
Herold, M.J., van den Brandt, J., Seibler, J. & Reichardt, H.M. Inducible and reversible gene silencing by stable integration of an shRNA-encoding lentivirus in transgenic rats. Proc. Natl. Acad. Sci. USA 105, 18507–18512 (2008).
Geng, H., Wittwer, T., Dittrich-Breiholz, O., Kracht, M. & Schmitz, M.L. Phosphorylation of NF-κB p65 at Ser468 controls its COMMD1-dependent ubiquitination and target gene-specific proteasomal elimination. EMBO Rep. 10, 381–386 (2009).
Schwabe, R.F. & Sakurai, H. IKKβ phosphorylates p65 at S468 in transactivaton domain 2. FASEB J. 19, 1758–1760 (2005).
Tanaka, T., Grusby, M.J. & Kaisho, T. PDLIM2-mediated termination of transcription factor NF-κB activation by intranuclear sequestration and degradation of the p65 subunit. Nat. Immunol. 8, 584–591 (2007).
Saccani, S., Marazzi, I., Beg, A.A. & Natoli, G. Degradation of promoter-bound p65/RelA is essential for the prompt termination of the nuclear factor κB response. J. Exp. Med. 200, 107–113 (2004).
Ryo, A. et al. Regulation of NF-κB signaling by Pin1-dependent prolyl isomerization and ubiquitin-mediated proteolysis of p65/RelA. Mol. Cell 12, 1413–1426 (2003).
Maine, G.N., Mao, X., Komarck, C.M. & Burstein, E. COMMD1 promotes the ubiquitination of NF-κB subunits through a cullin-containing ubiquitin ligase. EMBO J. 26, 436–447 (2007).
Ruland, J. et al. Bcl10 is a positive regulator of antigen receptor-induced activation of NF-κB and neural tube closure. Cell 104, 33–42 (2001).
Pasparakis, M., Alexopoulou, L., Episkopou, V. & Kollias, G. Immune and inflammatory responses in TNFα-deficient mice: a critical requirement for TNFα in the formation of primary B cell follicles, follicular dendritic cell networks and germinal centers, and in the maturation of the humoral immune response. J. Exp. Med. 184, 1397–1411 (1996).
Dalton, D.K. et al. Multiple defects of immune cell function in mice with disrupted interferon-γ genes. Science 259, 1739–1742 (1993).
Hou, B., Reizis, B. & DeFranco, A.L. Toll-like receptors activate innate and adaptive immunity by using dendritic cell-intrinsic and -extrinsic mechanisms. Immunity 29, 272–282 (2008).
Wijsman, J.H. et al. A new method to detect apoptosis in paraffin sections: in situ end-labeling of fragmented DNA. J. Histochem. Cytochem. 41, 7–12 (1993).
Bonnard, M. et al. Deficiency of T2K leads to apoptotic liver degeneration and impaired NF-κB-dependent gene transcription. EMBO J. 19, 4976–4985 (2000).
Calzascia, T. et al. TNF-α is critical for antitumor but not antiviral T cell immunity in mice. J. Clin. Invest. 117, 3833–3845 (2007).
Esashi, E. et al. The signal transducer STAT5 inhibits plasmacytoid dendritic cell development by suppressing transcription factor IRF8. Immunity 28, 509–520 (2008).
Pellegrini, M. et al. Adjuvant IL-7 antagonizes multiple cellular and molecular inhibitory networks to enhance immunotherapies. Nat Med 15, 528–536 (2009).
Acknowledgements
We thank J. Haight for animal care; A. Elia, L. Zhou and K. So and M. Macasaet for assistance with histopathology; R. Nayyar and F. Tong for flow cytometry support; M. Herold for assistance with the transduction of retroviruses, lentiviruses and short hairpin RNA; and T. Calzascia, C. Deluca, Y.J. Yang, A. Cheung, J. Woodgett, V. Stambolic and M. Minden for discussions. Supported by the Terry Fox Cancer Foundation (T.W.M. and P.S.O.), the National Cancer Institute of Canada (T.W.M. and P.S.O.), Canada Research Chairs (P.S.O.), the Cancer Research Institute (M.P.), National Health and Medical Research Council Australia (Career Development Award 637350 to M.P.), Victorian State Government Operational Infrastructure Support (M.P.), the Independent Research Institutes Infrastructure Support Scheme of the Australian Government National Health and Medical Research Council (M.P.) and the Canadian Institutes of Health Research (A.E.L.).
Author information
Authors and Affiliations
Contributions
A.E.L., M.P., G.E., Y.O., M.S., P.S.O. and T.W.M. designed and did all of the research with assistance from S.P.P., J.G.T., J.P.C., H.W.S., S.D.S., D.D. and R.H.K. and technical assistance from A.W., A.S., G.D., A.Y.-T., and J.S.; A.E.L. wrote the manuscript; and T.W.M. and M.P. directed the project and contributed to the preparation of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–4 and Tables 1–5 (PDF 485 kb)
Rights and permissions
About this article
Cite this article
Lin, A., Ebert, G., Ow, Y. et al. ARIH2 is essential for embryogenesis, and its hematopoietic deficiency causes lethal activation of the immune system. Nat Immunol 14, 27–33 (2013). https://doi.org/10.1038/ni.2478
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.2478