Abstract
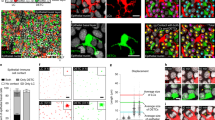
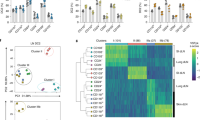
Langerhans cells (LCs) are epidermal dendritic cells with incompletely understood origins that associate with hair follicles for unknown reasons. Here we show that in response to external stress, mouse hair follicles recruited Gr-1hi monocyte-derived precursors of LCs whose epidermal entry was dependent on the chemokine receptors CCR2 and CCR6, whereas the chemokine receptor CCR8 inhibited the recruitment of LCs. Distinct hair-follicle regions had differences in their expression of ligands for CCR2 and CCR6. The isthmus expressed the chemokine CCL2; the infundibulum expressed the chemokine CCL20; and keratinocytes in the bulge produced the chemokine CCL8, which is the ligand for CCR8. Thus, distinct hair-follicle keratinocyte subpopulations promoted or inhibited repopulation with LCs via differences in chemokine production, a feature also noted in humans. Pre-LCs failed to enter hairless skin in mice or humans, which establishes hair follicles as portals for LCs.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Cotsarelis, G., Sun, T.T. & Lavker, R.M. Label-retaining cells reside in the bulge area of pilosebaceous unit: implications for follicular stem cells, hair cycle, and skin carcinogenesis. Cell 61, 1329–1337 (1990).
Lyle, S. et al. The C8/144B monoclonal antibody recognizes cytokeratin 15 and defines the location of human hair follicle stem cells. J. Cell Sci. 111, 3179–3188 (1998).
Trempus, C.S. et al. Enrichment for living murine keratinocytes from the hair follicle bulge with the cell surface marker CD34. J. Invest. Dermatol. 120, 501–511 (2003).
Christoph, T. et al. The human hair follicle immune system: cellular composition and immune privilege. Br. J. Dermatol. 142, 862–873 (2000).
Meyer, K.C. et al. Evidence that the bulge region is a site of relative immune privilege in human hair follicles. Br. J. Dermatol. 159, 1077–1085 (2008).
Hoek, R.M. et al. Down-regulation of the macrophage lineage through interaction with OX2 (CD200). Science 290, 1768–1771 (2000).
Stefanato, C.M. Histopathology of alopecia: a clinicopathological approach to diagnosis. Histopathology 56, 24–38 (2010).
Merad, M. et al. Langerhans cells renew in the skin throughout life under steady-state conditions. Nat. Immunol. 3, 1135–1141 (2002).
Borkowski, T.A., Nelson, A.J., Farr, A.G. & Udey, M.C. Expression of gp40, the murine homologue of human epithelial cell adhesion molecule (Ep-CAM), by murine dendritic cells. Eur. J. Immunol. 26, 110–114 (1996).
Bursch, L.S. et al. Identification of a novel population of Langerin+ dendritic cells. J. Exp. Med. 204, 3147–3156 (2007).
Nagao, K. et al. Murine epidermal Langerhans cells and langerin-expressing dermal dendritic cells are unrelated and exhibit distinct functions. Proc. Natl. Acad. Sci. USA 106, 3312–3317 (2009).
Borkowski, T.A., Letterio, J.J., Farr, A.G. & Udey, M.C. A role for endogenous transforming growth factor β1 in Langerhans cell biology: the skin of transforming growth factor β1 null mice is devoid of epidermal Langerhans cells. J. Exp. Med. 184, 2417–2422 (1996).
Kaplan, D.H. et al. Autocrine/paracrine TGFβ1 is required for the development of epidermal Langerhans cells. J. Exp. Med. 204, 2545–2552 (2007).
Zahner, S.P. et al. Conditional deletion of TGF-βR1 using Langerin-Cre mice results in Langerhans cell deficiency and reduced contact hypersensitivity. J. Immunol. 187, 5069–5076 (2011).
Igyártó, B.Z. et al. Skin-resident murine dendritic cell subsets promote distinct and opposing antigen-specific T helper cell responses. Immunity 35, 260–272 (2011).
Ouchi, T. et al. Langerhans cell antigen capture through tight junctions confers preemptive immunity in experimental staphylococcal scalded skin syndrome. J. Exp. Med. 208, 2607–2613 (2011).
Ginhoux, F. et al. Langerhans cells arise from monocytes in vivo. Nat. Immunol. 7, 265–273 (2006).
Chorro, L. et al. Langerhans cell (LC) proliferation mediates neonatal development, homeostasis, and inflammation-associated expansion of the epidermal LC network. J. Exp. Med. 206, 3089–3100 (2009).
Mantovani, A., Bonecchi, R. & Locati, M. Tuning inflammation and immunity by chemokine sequestration: decoys and more. Nat. Rev. Immunol. 6, 907–918 (2006).
Förster, R. et al. CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell 99, 23–33 (1999).
Ohl, L. et al. CCR7 governs skin dendritic cell migration under inflammatory and steady-state conditions. Immunity 21, 279–288 (2004).
Kabashima, K. et al. CXCL12-CXCR4 engagement is required for migration of cutaneous dendritic cells. Am. J. Pathol. 171, 1249–1257 (2007).
Merad, M. et al. Depletion of host Langerhans cells before transplantation of donor alloreactive T cells prevents skin graft-versus-host disease. Nat. Med. 10, 510–517 (2004).
Bennett, C.L. et al. Inducible ablation of mouse Langerhans cells diminishes but fails to abrogate contact hypersensitivity. J. Cell Biol. 169, 569–576 (2005).
Auffray, C. et al. Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science 317, 666–670 (2007).
Cheong, C. et al. Microbial stimulation fully differentiates monocytes to DC-SIGN/CD209+ dendritic cells for immune T cell areas. Cell 143, 416–429 (2011).
Clausen, B.E., Burkhardt, C., Reith, W., Renkawitz, R. & Förster, I. Conditional gene targeting in macrophages and granulocytes using LysMcre mice. Transgenic Res. 8, 265–277 (1999).
Kawamoto, S. et al. A novel reporter mouse strain that expresses enhanced green fluorescent protein upon Cre-mediated recombination. FEBS Lett. 470, 263–268 (2000).
Mian, A. et al. Toxicity and adaptive immune response to intracellular transgenes delivered by helper-dependent vs. first generation adenoviral vectors. Mol. Genet. Metab. 84, 278–288 (2005).
Jensen, U.B. et al. A distinct population of clonogenic and multipotent murine follicular keratinocytes residing in the upper isthmus. J. Cell Sci. 121, 609–617 (2008).
Nagao, K. et al. Abnormal placental development and early embryonic lethality in EpCAM-null mice. PLoS ONE 4, e8543 (2009).
Nishimura, E.K. et al. Dominant role of the niche in melanocyte stem-cell fate determination. Nature 416, 854–860 (2002).
Matloubian, M. et al. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature 427, 355–360 (2004).
Schwab, S.R. & Cyster, J.G. Finding a way out: lymphocyte egress from lymphoid organs. Nat. Immunol. 8, 1295–1301 (2007).
Ishii, M. et al. Sphingosine-1-phosphate mobilizes osteoclast precursors and regulates bone homeostasis. Nature 458, 524–528 (2009).
Liu, Y. et al. Edg-1, the G protein-coupled receptor for sphingosine-1-phosphate, is essential for vascular maturation. J. Clin. Invest. 106, 951–961 (2000).
Horiuchi, K. et al. Conditional inactivation of TACE by a Sox9 promoter leads to osteoporosis and increased granulopoiesis via dysregulation of IL-17 and G-CSF. J. Immunol. 182, 2093–2101 (2009).
Kobayashi, T., Iwasaki, T., Amagai, M. & Ohyama, M. Canine follicle stem cell candidates reside in the bulge and share characteristic features with human bulge cells. J. Invest. Dermatol. 130, 1988–1995 (2010).
Ghoreishi, M., Martinka, M. & Dutz, J.P. Type 1 interferon signature in the scalp lesions of alopecia areata. Br. J. Dermatol. 163, 57–62 (2010).
Gilliam, A.C. et al. The human hair follicle: a reservoir of CD40+ B7-deficient Langerhans cells that repopulate epidermis after UVB exposure. J. Invest. Dermatol. 110, 422–427 (1998).
Paus, R. et al. Generation and cyclic remodeling of the hair follicle immune system in mice. J. Invest. Dermatol. 111, 7–18 (1998).
Ito, M. et al. Stem cells in the hair follicle bulge contribute to wound repair but not to homeostasis of the epidermis. Nat. Med. 11, 1351–1354 (2005).
Clark, R.A. et al. The vast majority of CLA+ T cells are resident in normal skin. J. Immunol. 176, 4431–4439 (2006).
Chensue, S.W. et al. Aberrant in vivo T helper type 2 cell response and impaired eosinophil recruitment in CC chemokine receptor 8 knockout mice. J. Exp. Med. 193, 573–584 (2001).
Hedrick, M.N. et al. CCR6 is required for IL-23-induced psoriasis-like inflammation in mice. J. Clin. Invest. 119, 2317–2329 (2009).
Murai, M. et al. Peyer's patch is the essential site in initiating murine acute and lethal graft-versus-host reaction. Nat. Immunol. 4, 154–160 (2003).
Morikawa, T. et al. Hypoxic regulation of the cerebral microcirculation is mediated by a carbon monoxide-sensitive hydrogen sulfide pathway. Proc. Natl. Acad. Sci. USA 109, 1293–1298 (2012).
Belsito, D.V., Baer, R.L., Schultz, J.M. & Thorbecke, G.J. Relative lack of systemic effects of mometasone furoate on Langerhans cells of mice after topical administration as compared with other glucocorticosteroids. J. Invest. Dermatol. 91, 219–223 (1988).
Acknowledgements
We thank K. Eguchi and H. Ito for assistance, and M. Kajimura for assistance with multiphoton microscopy. Supported by the Japan Society for the Promotion of Science (K.N. and M.A.), The Kanae Foundation for the Promotion of Medical Science, and Japaneses Dermatological Association (K.N.), The Netherlands Organization for Scientific Research (B.E.C.) and the Intramural Research Program of the Center for Cancer Research of the National Cancer Institute (M.C.U.).
Author information
Authors and Affiliations
Contributions
K.N. conceived of and designed all experiments; K.N. and T.K. did experiments, with the assistance of K.Mo., T.A., D.Y.M., S.U., K.H., M.O., A.K. and Y.C.; B.E.C. provided Langerin-DTR mice; K.Ma. provided bone marrow from mice deficient in CCR1, CCR2 or CCR5 and Cx3cr1gfp/gfp mice; G.C.F. and S.A.L. provided bone marrow from CCR8-deficient mice; J.M.F. provided bone marrow from CCR6-deficient mice; H.T., K.K. and M.S. assisted with multiphoton intravital microscopy; K.Mo. assisted with the sorting of hair-follicle keratinocytes; M.C.U. and M.A. interpreted data and guided the project; and K.N. and M.C.U. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–4 and Table 1 (PDF 556 kb)
Supplementary Video 1
Multi-photon microscopy of DC recruitment to skin in vivo. (MOV 12159 kb)
Supplementary Video 2
Multi-photon microscopy of pre-LCs repopulation via HFs. (MOV 52721 kb)
Rights and permissions
About this article
Cite this article
Nagao, K., Kobayashi, T., Moro, K. et al. Stress-induced production of chemokines by hair follicles regulates the trafficking of dendritic cells in skin. Nat Immunol 13, 744–752 (2012). https://doi.org/10.1038/ni.2353
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.2353
This article is cited by
-
Developmentally programmed early-age skin localization of iNKT cells supports local tissue development and homeostasis
Nature Immunology (2023)
-
Time-course RNA-seq analysis reveals stage-specific and melatonin-triggered gene expression patterns during the hair follicle growth cycle in Capra hircus
BMC Genomics (2022)
-
Skin immunity: dissecting the complex biology of our body's outer barrier
Mucosal Immunology (2022)
-
Low-level laser treatment promotes skin wound healing by activating hair follicle stem cells in female mice
Lasers in Medical Science (2022)
-
Skin-resident immune cells actively coordinate their distribution with epidermal cells during homeostasis
Nature Cell Biology (2021)