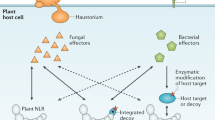
Abstract
In plants and animals, the NLR family of receptors perceives non-self and modified-self molecules inside host cells and mediates innate immune responses to microbial pathogens. Despite their similar biological functions and protein architecture, animal NLRs are normally activated by conserved microbe- or damage-associated molecular patterns, whereas plant NLRs typically detect strain-specific pathogen effectors. Plant NLRs recognize either the effector structure or effector-mediated modifications of host proteins. The latter indirect mechanism for the perception of non-self, as well as the within-species diversification of plant NLRs, maximize the capacity to recognize non-self through the use of a finite number of innate immunoreceptors. We discuss recent insights into NLR activation, signal initiation through the homotypic association of N-terminal domains and subcellular receptor dynamics in plants and compare those with NLR functions in animals.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Katie Vicari


Similar content being viewed by others
References
Boller, T. & Felix, G. A renaissance of elicitors: perception of microbe-associated molecular patterns and danger signals by pattern-recognition receptors. Annu. Rev. Plant Biol. 60, 379–406 (2009).
Jones, J.D. & Dangl, J.L. The plant immune system. Nature 444, 323–329 (2006).
Ausubel, F.M. Are innate immune signaling pathways in plants and animals conserved? Nat. Immunol. 6, 973–979 (2005).
Leipe, D.D., Koonin, E.V. & Aravind, L. STAND, a class of P-loop NTPases including animal and plant regulators of programmed cell death: multiple, complex domain architectures, unusual phyletic patterns, and evolution by horizontal gene transfer. J. Mol. Biol. 343, 1–28 (2004).
Li, J. et al. Unique evolutionary pattern of numbers of gramineous NBS-LRR genes. Mol. Genet. Genomics 283, 427–438 (2010).
Meyers, B.C., Kozik, A., Griego, A., Kuang, H. & Michelmore, R.W. Genome-wide analysis of NBS-LRR-encoding genes in Arabidopsis. Plant Cell 15, 809–834 (2003).
Lange, C. et al. Defining the origins of the NOD-like receptor system at the base of animal evolution. Mol. Biol. Evol. 28, 1687–1702 (2011).
Zhang, Q., Zmasek, C.M. & Godzik, A. Domain architecture evolution of pattern-recognition receptors. Immunogenetics 62, 263–272 (2010).
Rast, J.P., Smith, L.C., Loza-Coll, M., Hibino, T. & Litman, G.W. Genomic insights into the immune system of the sea urchin. Science 314, 952–956 (2006).
Hibino, T. et al. The immune gene repertoire encoded in the purple sea urchin genome. Dev. Biol. 300, 349–365 (2006).
Stein, C., Caccamo, M., Laird, G. & Leptin, M. Conservation and divergence of gene families encoding components of innate immune response systems in zebrafish. Genome Biol. 8, R251 (2007).
Coll, N.S., Epple, P. & Dangl, J.L. Programmed cell death in the plant immune system. Cell Death Differ. 18, 1247–1256 (2011).
Pieterse, C.M., Leon-Reyes, A., Van der Ent, S. & Van Wees, S.C. Networking by small-molecule hormones in plant immunity. Nat. Chem. Biol. 5, 308–316 (2009).
Ryan, C.A., Huffaker, A. & Yamaguchi, Y. New insights into innate immunity in Arabidopsis. Cell. Microbiol. 9, 1902–1908 (2007).
Bryan, G.T. et al. tA single amino acid difference distinguishes resistant and susceptible alleles of the rice blast resistance gene Pi-ta. Plant Cell 12, 2033–2046 (2000).
Jia, Y., McAdams, S.A., Bryan, G.T., Hershey, H.P. & Valent, B. Direct interaction of resistance gene and avirulence gene products confers rice blast resistance. EMBO J. 19, 4004–4014 (2000).
Dodds, P.N. et al. Direct protein interaction underlies gene-for-gene specificity and coevolution of the flax resistance genes and flax rust avirulence genes. Proc. Natl. Acad. Sci. USA 103, 8888–8893 (2006).
Deslandes, L. et al. Physical interaction between RRS1-R, a protein conferring resistance to bacterial wilt, and PopP2, a type III effector targeted to the plant nucleus. Proc. Natl. Acad. Sci. USA 100, 8024–8029 (2003).
Krasileva, K.V., Dahlbeck, D. & Staskawicz, B.J. Activation of an Arabidopsis resistance protein is specified by the in planta association of its leucine-rich repeat domain with the cognate oomycete effector. Plant Cell 22, 2444–2458 (2010).
Kuang, H., Caldwell, K.S., Meyers, B.C. & Michelmore, R.W. Frequent sequence exchanges between homologs of RPP8 in Arabidopsis are not necessarily associated with genomic proximity. Plant J. 54, 69–80 (2008).
Rehmany, A.P. et al. Differential recognition of highly divergent downy mildew avirulence gene alleles by RPP1 resistance genes from two Arabidopsis lines. Plant Cell 17, 1839–1850 (2005).
Rose, L.E. et al. The maintenance of extreme amino acid diversity at the disease resistance gene, RPP13, in Arabidopsis thaliana. Genetics 166, 1517–1527 (2004).
Ellis, J.G., Lawrence, G.J., Luck, J.E. & Dodds, P.N. Identification of regions in alleles of the flax rust resistance gene L that determine differences in gene-for-gene specificity. Plant Cell 11, 495–506 (1999).
Lawrence, G.J., Anderson, P.A., Dodds, P.N. & Ellis, J.G. Relationships between rust resistance genes at the M locus in flax. Mol. Plant Pathol. 11, 19–32 (2010).
Bhullar, N.K., Zhang, Z., Wicker, T. & Keller, B. Wheat gene bank accessions as a source of new alleles of the powdery mildew resistance gene Pm3: a large scale allele mining project. BMC Plant Biol. 10, 88 (2010).
Seeholzer, S. et al. Diversity at the Mla powdery mildew resistance locus from cultivated barley reveals sites of positive selection. Mol. Plant Microbe Interact. 23, 497–509 (2010).
Shen, Q.H. et al. Recognition specificity and RAR1/SGT1 dependence in barley Mla disease resistance genes to the powdery mildew fungus. Plant Cell 15, 732–744 (2003).
Yahiaoui, N., Brunner, S. & Keller, B. Rapid generation of new powdery mildew resistance genes after wheat domestication. Plant J. 47, 85–98 (2006).
Wang, C.I. et al. Crystal structures of flax rust avirulence proteins AvrL567-A and -D reveal details of the structural basis for flax disease resistance specificity. Plant Cell 19, 2898–2912 (2007).
Ravensdale, M., Nemri, A., Thrall, P.H., Ellis, J.G. & Dodds, P.N. Co-evolutionary interactions between host resistance and pathogen effector genes in flax rust disease. Mol. Plant Pathol. 12, 93–102 (2011).
Botella, M.A. et al. Three genes of the Arabidopsis RPP1 complex resistance locus recognize distinct Peronospora parasitica avirulence determinants. Plant Cell 10, 1847–1860 (1998).
McDowell, J.M. et al. Intragenic recombination and diversifying selection contribute to the evolution of downy mildew resistance at the RPP8 locus of Arabidopsis. Plant Cell 10, 1861–1874 (1998).
Meyers, B.C. et al. The major resistance gene cluster in lettuce is highly duplicated and spans several megabases. Plant Cell 10, 1817–1832 (1998).
Girardin, S.E. et al. Identification of the critical residues involved in peptidoglycan detection by Nod1. J. Biol. Chem. 280, 38648–38656 (2005).
Hu, Y., Benedict, M.A., Ding, L. & Nunez, G. Role of cytochrome c and dATP/ATP hydrolysis in Apaf-1-mediated caspase-9 activation and apoptosis. EMBO J. 18, 3586–3595 (1999).
Yu, X. et al. A structure of the human apoptosome at 12.8 A resolution provides insights into this cell death platform. Structure 13, 1725–1735 (2005).
Baxter, L. et al. Signatures of adaptation to obligate biotrophy in the Hyaloperonospora arabidopsidis genome. Science 330, 1549–1551 (2010).
Haas, B.J. et al. Genome sequence and analysis of the Irish potato famine pathogen Phytophthora infestans. Nature 461, 393–398 (2009).
Schirawski, J. et al. Pathogenicity determinants in smut fungi revealed by genome comparison. Science 330, 1546–1548 (2010).
Raffaele, S. et al. Genome evolution following host jumps in the Irish potato famine pathogen lineage. Science 330, 1540–1543 (2010).
Spanu, P.D. et al. Genome expansion and gene loss in powdery mildew fungi reveal tradeoffs in extreme parasitism. Science 330, 1543–1546 (2010).
Dangl, J.L. & Jones, J.D. Plant pathogens and integrated defence responses to infection. Nature 411, 826–833 (2001).
van der Hoorn, R.A. & Kamoun, S. From guard to decoy: a new model for perception of plant pathogen effectors. Plant Cell 20, 2009–2017 (2008).
Caplan, J.L., Mamillapalli, P., Burch-Smith, T.M., Czymmek, K. & Dinesh-Kumar, S.P. Chloroplastic protein NRIP1 mediates innate immune receptor recognition of a viral effector. Cell 132, 449–462 (2008).
Collier, S.M. & Moffett, P. NB-LRRs work a “bait and switch” on pathogens. Trends Plant Sci. 14, 521–529 (2009).
Xiang, T. et al. Pseudomonas syringae effector AvrPto blocks innate immunity by targeting receptor kinases. Curr. Biol. 18, 74–80 (2008).
Shan, L. et al. Bacterial effectors target the common signaling partner BAK1 to disrupt multiple MAMP receptor-signaling complexes and impede plant immunity. Cell Host Microbe 4, 17–27 (2008).
Xing, W. et al. The structural basis for activation of plant immunity by bacterial effector protein AvrPto. Nature 449, 243–247 (2007).
Martin, G.B. et al. Map-based cloning of a protein kinase gene conferring disease resistance in tomato. Science 262, 1432–1436 (1993).
Rathjen, J.P., Chang, J.H., Staskawicz, B.J. & Michelmore, R.W. Constitutively active Pto induces a Prf-dependent hypersensitive response in the absence of avrPto. EMBO J. 18, 3232–3240 (1999).
Gutierrez, J.R. et al. Prf immune complexes of tomato are oligomeric and contain multiple Pto-like kinases that diversify effector recognition. Plant J. 61, 507–518 (2010).
Grant, M.R. et al. Structure of the Arabidopsis RPM1 gene enabling dual specificity disease resistance. Science 269, 843–846 (1995).
Chung, E.H. et al. Specific threonine phosphorylation of a host target by two unrelated type III effectors activates a host innate immune receptor in plants. Cell Host Microbe 9, 125–136 (2011).
Liu, J., Elmore, J.M., Lin, Z.J. & Coaker, G.A. Receptor-like cytoplasmic kinase phosphorylates the host target RIN4, leading to the activation of a plant innate immune Receptor. Cell Host Microbe 9, 137–146 (2011).
Mackey, D., Holt, B.F. III, Wiig, A. & Dangl, J.L. RIN4 interacts with Pseudomonas syringae type III effector molecules and is required for RPM1-mediated resistance in Arabidopsis. Cell 108, 743–754 (2002).
Axtell, M.J. & Staskawicz, B.J. Initiation of RPS2-specified disease resistance in Arabidopsis is coupled to the AvrRpt2-directed elimination of RIN4. Cell 112, 369–377 (2003).
Mackey, D., Belkhadir, Y., Alonso, J.M., Ecker, J.R. & Dangl, J.L. Arabidopsis RIN4 is a target of the type III virulence effector AvrRpt2 and modulates RPS2-mediated resistance. Cell 112, 379–389 (2003).
Schroder, K. & Tschopp, J. The inflammasomes. Cell 140, 821–832 (2010).
Diez, E. et al. Birc1e is the gene within the Lgn1 locus associated with resistance to Legionella pneumophila. Nat. Genet. 33, 55–60 (2003).
Steinfeldt, T. et al. Phosphorylation of mouse immunity-related GTPase (IRG) resistance proteins is an evasion strategy for virulent Toxoplasma gondii. PLoS Biol. 8, e1000576 (2010).
Danot, O., Marquenet, E., Vidal-Ingigliardi, D. & Richet, E. Wheel of life, wheel of death: a mechanistic insight into signaling by STAND proteins. Structure 17, 172–182 (2009).
Proell, M., Riedl, S.J., Fritz, J.H., Rojas, A.M. & Schwarzenbacher, R. The Nod-like receptor (NLR) family: a tale of similarities and differences. PLoS ONE 3, e2119 (2008).
van Ooijen, G., van den Burg, H.A., Cornelissen, B.J. & Takken, F.L. Structure and function of resistance proteins in solanaceous plants. Annu. Rev. Phytopathol. 45, 43–72 (2007).
Takken, F.L., Albrecht, M. & Tameling, W.I. Resistance proteins: molecular switches of plant defence. Curr. Opin. Plant Biol. 9, 383–390 (2006).
Rosenstiel, P., Till, A. & Schreiber, S. NOD-like receptors and human diseases. Microbes Infect. 9, 648–657 (2007).
Zurek, B., Proell, M., Wagner, R.N., Schwarzenbacher, R. & Kufer, T.A. Mutational analysis of human NOD1 and NOD2 NACHT domains reveals different modes of activation. Innate Immun. published online, doi:10.1177/1753425910394002 (10 February 2011).
Moffett, P., Farnham, G., Peart, J. & Baulcombe, D.C. Interaction between domains of a plant NBS-LRR protein in disease resistance-related cell death. EMBO J. 21, 4511–4519 (2002).
Bendahmane, A., Farnham, G., Moffett, P. & Baulcombe, D.C. Constitutive gain-of-function mutants in a nucleotide binding site-leucine rich repeat protein encoded at the Rx locus of potato. Plant J. 32, 195–204 (2002).
Acehan, D. et al. Three-dimensional structure of the apoptosome: implications for assembly, procaspase-9 binding, and activation. Mol. Cell 9, 423–432 (2002).
Riedl, S.J., Li, W., Chao, Y., Schwarzenbacher, R. & Shi, Y. Structure of the apoptotic protease-activating factor 1 bound to ADP. Nature 434, 926–933 (2005).
Tanabe, T. et al. Regulatory regions and critical residues of NOD2 involved in muramyl dipeptide recognition. EMBO J. 23, 1587–1597 (2004).
Ade, J., DeYoung, B.J., Golstein, C. & Innes, R.W. Indirect activation of a plant nucleotide binding site-leucine-rich repeat protein by a bacterial protease. Proc. Natl. Acad. Sci. USA 104, 2531–2536 (2007).
Shen, Q.H. & Schulze-Lefert, P. Rumble in the nuclear jungle: compartmentalization, trafficking, and nuclear action of plant immune receptors. EMBO J. 26, 4293–4301 (2007).
Coussens, N.P., Mowers, J.C., McDonald, C., Nunez, G. & Ramaswamy, S. Crystal structure of the Nod1 caspase activation and recruitment domain. Biochem. Biophys. Res. Commun. 353, 1–5 (2007).
Srimathi, T. et al. Monomer/dimer transition of the caspase-recruitment domain of human Nod1. Biochemistry 47, 1319–1325 (2008).
Qi, S. et al. Crystal structure of the Caenorhabditis elegans apoptosome reveals an octameric assembly of CED-4. Cell 141, 446–457 (2010).
Mestre, P. & Baulcombe, D.C. Elicitor-mediated oligomerization of the tobacco N disease resistance protein. Plant Cell 18, 491–501 (2006).
Bernoux, M. et al. Structural and functional analysis of a plant resistance protein TIR domain reveals interfaces for self-association, signaling, and autoregulation. Cell Host Microbe 9, 200–211 (2011).
Maekawa, T. et al. Coiled-coil domain-dependent homodimerization of intracellular barley immune receptors defines a minimal functional module for triggering cell death. Cell Host Microbe 9, 187–199 (2011).
Weaver, M.L., Swiderski, M.R., Li, Y. & Jones, J.D. The Arabidopsis thaliana TIR-NB-LRR R-protein, RPP1A; protein localization and constitutive activation of defence by truncated alleles in tobacco and Arabidopsis. Plant J. 47, 829–840 (2006).
Frost, D. et al. Tobacco transgenic for the flax rust resistance gene L expresses allele-specific activation of defense responses. Mol. Plant Microbe Interact. 17, 224–232 (2004).
Swiderski, M.R., Birker, D. & Jones, J.D. The TIR domain of TIR-NB-LRR resistance proteins is a signaling domain involved in cell death induction. Mol. Plant Microbe Interact. 22, 157–165 (2009).
Falk, A. et al. EDS1, an essential component of R gene-mediated disease resistance in Arabidopsis has homology to eukaryotic lipases. Proc. Natl. Acad. Sci. USA 96, 3292–3297 (1999).
Aarts, N. et al. Different requirements for EDS1 and NDR1 by disease resistance genes define at least two R gene-mediated signaling pathways in Arabidopsis. Proc. Natl. Acad. Sci. USA 95, 10306–10311 (1998).
Bertin, J. et al. Human CARD4 protein is a novel CED-4/Apaf-1 cell death family member that activates NF-kB. J. Biol. Chem. 274, 12955–12958 (1999).
Yuan, S. et al. Structure of an apoptosome-procaspase-9 CARD complex. Structure 18, 571–583 (2010).
Shirasu, K. The HSP90–SGT1 chaperone complex for NLR immune sensors. Annu. Rev. Plant Biol. 60, 139–164 (2009).
Holt, B.F. III, Belkhadir, Y. & Dangl, J.L. Antagonistic control of disease resistance protein stability in the plant immune system. Science 309, 929–932 (2005).
da Silva Correia, J., Miranda, Y., Leonard, N. & Ulevitch, R. SGT1 is essential for Nod1 activation. Proc. Natl. Acad. Sci. USA 104, 6764–6769 (2007).
Mayor, A., Martinon, F., De Smedt, T., Petrilli, V. & Tschopp, J. A crucial function of SGT1 and HSP90 in inflammasome activity links mammalian and plant innate immune responses. Nat. Immunol. 8, 497–503 (2007).
Khan, J.A., Brint, E.K., O'Neill, L.A. & Tong, L. Crystal structure of the Toll/interleukin-1 receptor domain of human IL-1RAPL. J. Biol. Chem. 279, 31664–31670 (2004).
Ohnishi, H. et al. Structural basis for the multiple interactions of the MyD88 TIR domain in TLR4 signaling. Proc. Natl. Acad. Sci. USA 106, 10260–10265 (2009).
Xu, Y. et al. Structural basis for signal transduction by the Toll/interleukin-1 receptor domains. Nature 408, 111–115 (2000).
Meyers, B.C., Morgante, M. & Michelmore, R.W. TIR-X and TIR-NBS proteins: two new families related to disease resistance TIR-NBS-LRR proteins encoded in Arabidopsis and other plant genomes. Plant J. 32, 77–92 (2002).
Dinesh-Kumar, S.P., Tham, W.H. & Baker, B.J. Structure-function analysis of the tobacco mosaic virus resistance gene N. Proc. Natl. Acad. Sci. USA 97, 14789–14794 (2000).
Chan, S.L., Mukasa, T., Santelli, E., Low, L.Y. & Pascual, J. The crystal structure of a TIR domain from Arabidopsis thaliana reveals a conserved helical region unique to plants. Protein Sci. 19, 155–161 (2010).
Tapping, R.I. Innate immune sensing and activation of cell surface Toll-like receptors. Semin. Immunol. 21, 175–184 (2009).
Rairdan, G.J. et al. The coiled-coil and nucleotide binding domains of the Potato Rx disease resistance protein function in pathogen recognition and signaling. Plant Cell 20, 739–751 (2008).
Mazourek, M. et al. The fractionated orthology of Bs2 and Rx/Gpa2 supports shared synteny of disease resistance in the Solanaceae. Genetics 182, 1351–1364 (2009).
Tornero, P., Chao, R.A., Luthin, W.N., Goff, S.A. & Dangl, J.L. Large-scale structure-function analysis of the Arabidopsis RPM1 disease resistance protein. Plant Cell 14, 435–450 (2002).
Hall, H.T. et al. RIP2 contributes to Nod signaling but is not essential for T cell proliferation, T helper differentiation or TLR responses. Eur. J. Immunol. 38, 64–72 (2008).
Mariathasan, S. et al. Differential activation of the inflammasome by caspase-1 adaptors ASC and Ipaf. Nature 430, 213–218 (2004).
Park, J.H. et al. RICK/RIP2 mediates innate immune responses induced through Nod1 and Nod2 but not TLRs. J. Immunol. 178, 2380–2386 (2007).
Manon, F., Favier, A., Nunez, G., Simorre, J.P. & Cusack, S. Solution structure of NOD1 CARD and mutational analysis of its interaction with the CARD of downstream kinase RICK. J. Mol. Biol. 365, 160–174 (2007).
Kufer, T.A. Signal transduction pathways used by NLR-type innate immune receptors. Mol. Biosyst. 4, 380–386 (2008).
Bauernfeind, F.G. et al. Cutting edge: NF-kB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J. Immunol. 183, 787–791 (2009).
Broz, P. et al. Redundant roles for inflammasome receptors NLRP3 and NLRC4 in host defense against Salmonella. J. Exp. Med. 207, 1745–1755 (2010).
Tameling, W.I. et al. RanGAP2 mediates nucleocytoplasmic partitioning of the NB-LRR immune receptor Rx in the Solanaceae, thereby dictating Rx function. Plant Cell 22, 4176–4194 (2010).
Slootweg, E. et al. Nucleocytoplasmic distribution is required for activation of resistance by the potato NB-LRR receptor Rx1 and is balanced by its functional domains. Plant Cell 22, 4195–4215 (2010).
García, A.V. et al. Balanced nuclear and cytoplasmic activities of EDS1 are required for a complete plant innate immune response. PLoS Pathog. 6, e1000970 (2010).
Wirthmueller, L., Zhang, Y., Jones, J.D. & Parker, J.E. Nuclear accumulation of the Arabidopsis immune receptor RPS4 is necessary for triggering EDS1-dependent defense. Curr. Biol. 17, 2023–2029 (2007).
Cheng, Y.T. et al. Nuclear pore complex component MOS7/Nup88 is required for innate immunity and nuclear accumulation of defense regulators in Arabidopsis. Plant Cell 21, 2503–2516 (2009).
Meissner, T.B. et al. NLR family member NLRC5 is a transcriptional regulator of MHC class I genes. Proc. Natl. Acad. Sci. USA 107, 13794–13799 (2010).
Hake, S.B. et al. CIITA leucine-rich repeats control nuclear localization, in vivo recruitment to the major histocompatibility complex (MHC) class II enhanceosome, and MHC class II gene transactivation. Mol. Cell. Biol. 20, 7716–7725 (2000).
Ting, J.P. & Trowsdale, J. Genetic control of MHC class II expression. Cell 109 Suppl, S21– S33 (2002).
Peart, J.R., Mestre, P., Lu, R., Malcuit, I. & Baulcombe, D.C. NRG1, a CC-NB-LRR protein, together with N, a TIR-NB-LRR protein, mediates resistance against tobacco mosaic virus. Curr. Biol. 15, 968–973 (2005).
Eitas, T.K., Nimchuk, Z.L. & Dangl, J.L. Arabidopsis TAO1 is a TIR-NB-LRR protein that contributes to disease resistance induced by the Pseudomonas syringae effector AvrB. Proc. Natl. Acad. Sci. USA 105, 6475–6480 (2008).
Narusaka, M. et al. RRS1 and RPS4 provide a dual Resistance-gene system against fungal and bacterial pathogens. Plant J. 60, 218–226 (2009).
Eitas, T.K. & Dangl, J.L. NB-LRR proteins: pairs, pieces, perception, partners, and pathways. Curr. Opin. Plant Biol. 13, 472–477 (2010).
Damiano, J.S., Oliveira, V., Welsh, K. & Reed, J.C. Heterotypic interactions among NACHT domains: implications for regulation of innate immune responses. Biochem. J. 381, 213–219 (2004).
Pan, Q. et al. MDP-induced interleukin-1β processing requires Nod2 and CIAS1/NALP3. J. Leukoc. Biol. 82, 177–183 (2007).
Hsu, L.C. et al. A NOD2-NALP1 complex mediates caspase-1-dependent IL-1beta secretion in response to Bacillus anthracis infection and muramyl dipeptide. Proc. Natl. Acad. Sci. USA 105, 7803–7808 (2008).
Kwon, S.I., Kim, S.H., Bhattacharjee, S., Noh, J.J. & Gassmann, W. SRFR1, a suppressor of effector-triggered immunity, encodes a conserved tetratricopeptide repeat protein with similarity to transcriptional repressors. Plant J. 57, 109–119 (2009).
Kim, S.H. et al. The Arabidopsis resistance-like gene SNC1 is activated by mutations in SRFR1 and contributes to resistance to the bacterial effector AvrRps4. PLoS Pathog. 6, e1001172 (2010).
Li, Y. et al. SRFR1 negatively regulates plant NB-LRR resistance protein accumulation to prevent autoimmunity. PLoS Pathog. 6, e1001111 (2010).
Nishimura, M.T. & Dangl, J.L. Arabidopsis and the plant immune system. Plant J. 61, 1053–1066 (2010).
Gassmann, W. Natural variation in the Arabidopsis response to the avirulence gene hopPsyA uncouples the hypersensitive response from disease resistance. Mol. Plant Microbe Interact. 18, 1054–1060 (2005).
Bulgarelli, D. et al. The CC-NB-LRR-type Rdg2a resistance gene confers immunity to the seed-borne barley leaf stripe pathogen in the absence of hypersensitive cell death. PLoS ONE 5, e12599 (2010).
Coll, N.S. et al. Arabidopsis type I metacaspases control cell death. Science 330, 1393–1397 (2010).
Bendahmane, A., Kanyuka, K. & Baulcombe, D.C. The Rx gene from potato controls separate virus resistance and cell death responses. Plant Cell 11, 781–792 (1999).
Century, K.S., Holub, E.B. & Staskawicz, B.J. NDR1, a locus of Arabidopsis thaliana that is required for disease resistance to both a bacterial and a fungal pathogen. Proc. Natl. Acad. Sci. USA 92, 6597–6601 (1995).
Miao, E.A. et al. Caspase-1-induced pyroptosis is an innate immune effector mechanism against intracellular bacteria. Nat. Immunol. 11, 1136–1142 (2010).
Chen, Y., Smith, M.R., Thirumalai, K. & Zychlinsky, A. A bacterial invasin induces macrophage apoptosis by binding directly to ICE. EMBO J. 15, 3853–3860 (1996).
Govrin, E.M. & Levine, A. The hypersensitive response facilitates plant infection by the necrotrophic pathogen Botrytis cinerea. Curr. Biol. 10, 751–757 (2000).
Tsuda, K., Sato, M., Stoddard, T., Glazebrook, J. & Katagiri, F. Network properties of robust immunity in plants. PLoS Genet. 5, e1000772 (2009).
Rietz, S. et al. Different roles of Enhanced Disease Susceptibility1 (EDS1) bound to and dissociated from Phytoalexin Deficient4 (PAD4) in Arabidopsis immunity. New Phytol. 191, 107–119 (2011).
Dodds, P.N., Lawrence, G.J., Catanzariti, A.M., Ayliffe, M.A. & Ellis, J.G. The Melampsora lini AvrL567 avirulence genes are expressed in haustoria and their products are recognized inside plant cells. Plant Cell 16, 755–768 (2004).
Bittner-Eddy, P. et al. Genetic and physical mapping of the RPP13 locus, in Arabidopsis, responsible for specific recognition of several Peronospora parasitica (downy mildew) isolates. Mol. Plant Microbe Interact. 12, 792–802 (1999).
Allen, R.L. et al. Host-parasite coevolutionary conflict between Arabidopsis and downy mildew. Science 306, 1957–1960 (2004).
Hall, S.A. et al. Maintenance of genetic variation in plants and pathogens involves complex networks of gene-for-gene interactions. Mol. Plant Pathol. 10, 449–457 (2009).
Shen, Q.H. et al. Nuclear activity of MLA immune receptors links isolate-specific and basal disease-resistance responses. Science 315, 1098–1103 (2007).
Zhu, Z. et al. Arabidopsis resistance protein SNC1 activates immune responses through association with a transcriptional corepressor. Proc. Natl. Acad. Sci. USA 107, 13960–13965 (2010).
Zhang, Y. & Li, X. A putative nucleoporin 96 Is required for both basal defense and constitutive resistance responses mediated by suppressor of npr1–1,constitutive 1. Plant Cell 17, 1306–1316 (2005).
Palma, K., Zhang, Y. & Li, X. An importin alpha homolog, MOS6, plays an important role in plant innate immunity. Curr. Biol. 15, 1129–1135 (2005).
Acknowledgements
Supported by German Research Foundation in the collaborative research centre SFB670 (T.M., T.A.K. and P.S.-L.) and the Max Planck Society (P.S.-L.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Maekawa, T., Kufer, T. & Schulze-Lefert, P. NLR functions in plant and animal immune systems: so far and yet so close. Nat Immunol 12, 817–826 (2011). https://doi.org/10.1038/ni.2083
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.2083
This article is cited by
-
Commensal lifestyle regulated by a negative feedback loop between Arabidopsis ROS and the bacterial T2SS
Nature Communications (2024)
-
Genome-wide identification of nucleotide-binding domain leucine-rich repeat (NLR) genes and their association with green peach aphid (Myzus persicae) resistance in peach
BMC Plant Biology (2023)
-
MG1 interacts with a protease inhibitor and confers resistance to rice root-knot nematode
Nature Communications (2023)
-
Dying in self-defence: a comparative overview of immunogenic cell death signalling in animals and plants
Cell Death & Differentiation (2023)
-
Immune gene variation associated with chromosome-scale differences among individual zebrafish genomes
Scientific Reports (2023)