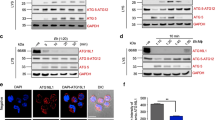
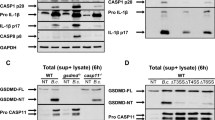
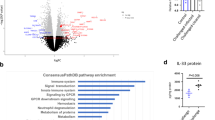
Abstract
Sepsis is one of the most challenging health problems worldwide. Here we found that phagocytes from patients with sepsis had considerable upregulation of Toll-like receptor 4 (TLR4) and TLR2; however, shock-inducing inflammatory responses mediated by these TLRs were inhibited by ES-62, an immunomodulator secreted by the filarial nematode Acanthocheilonema viteae. ES-62 subverted TLR4 signaling to block TLR2- and TLR4-driven inflammatory responses via autophagosome-mediated downregulation of the TLR adaptor-transducer MyD88. In vivo, ES-62 protected mice against endotoxic and polymicrobial septic shock by TLR4-mediated induction of autophagy and was protective even when administered after the induction of sepsis. Given that the treatments for septic shock at present are inadequate, the autophagy-dependent mechanism of action by ES-62 might form the basis for urgently needed therapeutic intervention against this life-threatening condition.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Change history
24 June 2011
The authors wish to note the following. Irregularities have been identified in some of the figures in this paper. The conclusions drawn from these data, that ES-62 protects against the development of pathology in the sepsis models and results in the induction of autophagy in macrophages, cannot be made. As these conclusions constitute major components of the paper, we wish to retract this paper.
References
Dombrovskiy, V.Y., Martin, A.A., Sunderram, J. & Paz, H.L. Rapid increase in hospitalization and mortality rates for severe sepsis in the United States: a trend analysis from 1993 to 2003. Crit. Care Med. 35, 1244–1250 (2007).
Padkin, A. et al. Epidemiology of severe sepsis occurring in the first 24 h in intensive care units in England, Wales, and Northern Ireland. Crit. Care Med. 31, 2332–2338 (2003).
Vincent, J.L. et al. The prevalence of nosocomial infection in intensive care units in Europe. Results of the European Prevalence of Infection in Intensive Care (EPIC) Study. EPIC International Advisory Committee. J. Am. Med. Assoc. 274, 639–644 (1995).
Martin, G.S., Mannino, D.M., Eaton, S. & Moss, M. The epidemiology of sepsis in the United States from 1979 through 2000. N. Engl. J. Med. 348, 1546–1554 (2003).
Balk, R.A. Severe sepsis and septic shock. Definitions, epidemiology, and clinical manifestations. Crit. Care Clin. 16, 179–192 (2000).
Alexander, C. & Rietschel, E.T. Bacterial lipopolysaccharides and innate immunity. J. Endotoxin Res. 7, 167–202 (2001).
Janeway, C.A. & Medzhitov, R. Innate immune recognition. Annu. Rev. Immunol. 20, 197–216 (2002).
Gay, N.J. & Gangloff, M. Structure and function of Toll receptors and their ligands. Annu. Rev. Biochem. 76, 141–165 (2007).
Doyle, S.L. & O'Neill, L.A. Toll-like receptors: from the discovery of NFκB to new insights into transcriptional regulations in innate immunity. Biochem. Pharmacol. 72, 1102–1113 (2006).
O'Neill, L.A. & Bowie, A.G. The family of five: TIR-domain-containing adaptors in Toll-like receptor signalling. Nat. Rev. Immunol. 7, 353–364 (2007).
Parrillo, J.E. et al. Septic shock in humans. Advances in the understanding of pathogenesis, cardiovascular dysfunction, and therapy. Ann. Intern. Med. 113, 227–242 (1990).
Exley, A.R., Leese, T., Holliday, M.P., Swann, R.A. & Cohen, J. Endotoxaemia and serum tumour necrosis factor as prognostic markers in severe acute pancreatitis. Gut 33, 1126–1128 (1992).
Wang, H. et al. HMG-1 as a late mediator of endotoxin lethality in mice. Science 285, 248–251 (1999).
Bustin, M. At the crossroads of necrosis and apoptosis, signaling to multiple cellular targets by HMGB1. Sci. STKE 151, PE39 (2002).
Andersson, U. et al. High mobility group 1 protein (HMG-1) stimulates proinflammatory cytokine synthesis in human monocytes. J. Exp. Med. 192, 565–570 (2000).
Goodridge, H.S. et al. Immunomodulation via novel use of TLR4 by the filarial nematode phosphorylcholine-containing secreted product, ES-62. J. Immunol. 174, 284–293 (2005).
Melendez, A.J. et al. Inhibition of FcɛRI-mediated mast cell responses by ES-62, a product of parasitic filarial nematodes. Nat. Med. 13, 1375–1381 (2007).
Salomão, R. et al. TLR signaling pathway in patients with sepsis. Shock 1, 73–77 (2008).
Weighardt, H. & Holzmann, B. Role of Toll-like receptor responses for sepsis pathogenesis. Immunobiology 212, 715–722 (2008).
Delgado, M.A. & Deretic, V. Toll-like receptors in control of immunological autophagy. Cell Death Differ. 16, 976–983 (2009).
Deretic, V. Multiple regulatory effector roles of autophagy in immunity. Curr. Opin. Immunol. 21, 53–62 (2009).
Klionsky, D.J. et al. Guidelines for the use and interpretation of assays for monitoring autophagy in higher eukaryotes. Autophagy 4, 151–175 (2008).
Mizushima, N. et al. Methods in mammalian autophagy research. Cell 1403, 313–326 (2010).
Phillip, D.R. & Parrillo, J.E. Mediator modulation therapy of severe sepsis and septic shock: does it work? Crit. Care Med. 32, 282–286 (2004).
Eichacker, P.Q. et al. Risk and the efficacy of antiinflammatory agents: retrospective and confirmatory studies of sepsis. Am. J. Respir. Crit. Care Med. 166, 1197–1205 (2002).
Hubbard, W.J. et al. Cecal ligation and puncture. Shock 1, 52–57 (2005).
Li, Y., Karlin, A., Loike, J.D. & Silverstein, S.C. A critical concentration of neutrophils is required for effective bacterial killing in suspension. Proc. Natl. Acad. Sci. USA 99, 8289–8294 (2002).
Weighardt, H. et al. Cutting edge: myeloid differentiation factor 88 deficiency improves resistance against sepsis caused by polymicrobial infection. J. Immunol. 169, 2823–2827 (2002).
Puneet, P. et al. SphK1 regulates proinflammatory responses associated with endotoxin and polymicrobial sepsis. Science 328, 1290–1294 (2010).
Sethu, S., Pushparaj, P.N. & Melendez, A.J. TNFα-induced inflammatory responses in vivo are mediated by PLD1. PLoS ONE 5, e10506 (2010).
Saitoh, T. et al. Loss of the autophagy protein Atg16L1 enhances endotoxin-induced IL-1beta production. Nature 456, 264–268 (2008).
Lee, H.-M. et al. Autophagy negatively regulates keratinocyte inflammatory responses via scaffolding protein p62/SQSTM1. J. Immunol. 186, 1248–1258 (2011).
Goodridge, H.S. et al. Modulation of macrophage cytokine production by ES-62, a secreted product of the filarial nematode Acanthocheilonema viteae. J. Immunol. 167, 940–945 (2001).
Henneke, P. et al. Cellular activation, phagocytosis, and bactericidal activity against group B streptococcus involve parallel myeloid differentiation factor 88-dependent and independent signaling pathways. J. Immunol. 169, 3970–3977 (2002).
Horng, T., Barton, G.M. & Medzhitov, R. TIRAP: an adapter molecule in the Toll signaling pathway. Nat. Immunol. 2, 835–841 (2001).
Horng, T. et al. The adaptor molecule TIRAP provides signalling specificity for Toll-like receptors. Nature 420, 329–333 (2002).
Doz, E. et al. Acylation determines the toll-like receptor (TLR)-dependent positive versus TLR2-, mannose receptor-, and SIGNR1-independent negative regulation of pro-inflammatory cytokines by mycobacterial lipomannan. J. Biol. Chem. 282, 26014–26025 (2007).
Ferwerda, B. et al. Functional and genetic evidence that the Mal/TIRAP allele variant 180L has been selected by providing protection against septic shock. Proc. Natl. Acad. Sci. USA 106, 10272–10277 (2009).
Daubeuf, B. et al. TLR4/MD-2 monoclonal antibody therapy affords protection in experimental models of septic shock. J. Immunol. 179, 6107–6114 (2007).
Sha, T. et al. Therapeutic effects of TAK-242, a novel selective Toll-like receptor 4 signal transduction inhibitor, in mouse endotoxin shock model. Eur. J. Pharmacol. 571, 231–239 (2007).
Leon, C.G., Tory, R., Jia, J., Sivak, O. & Wasan, K.M. Discovery and development of toll-like receptor 4 (TLR4) antagonists: a new paradigm for treating sepsis and other diseases. Pharm. Res. 25, 1751–1761 (2008).
Bone, R.C. et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis: members of the American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference Committee. Crit. Care Med. 20, 864–874 (1992).
McInnes, I.B. et al. A novel therapeutic approach targeting articular inflammation using the filarial nematode-derived phosphorylcholine-containing glycoprotein ES-62. J. Immunol. 171, 2127–2133 (2003).
Harnett, M.M. Laser scanning cytometry: understanding the immune system in situ. Nat. Rev. Immunol. 11, 897–904 (2007).
Morton, A.M. et al. Inverse Rap1 and phospho-ERK expression discriminate the maintenance phase of tolerance and priming of antigen-specific CD4+ T cells in vitro and in vivo. J. Immunol. 179, 8026–8034 (2007).
Melendez, A.J. & Ibrahim, F. Antisense knockdown of sphingosine kinase 1 in human macrophages inhibits C5a receptor-dependent signal transduction, Ca2+ signals, enzyme release, cytokine production and chemotaxis. J. Immunol. 173, 1596–1603 (2004).
Ibrahim, F.B., Pang, S.J. & Melendez, A.J. Anaphylatoxin signaling in human neutrophils: A key role for sphingosine kinase. J. Biol. Chem. 279, 44802–44811 (2004).
Acknowledgements
Supported by the Medical Research Council UK (G0700794), the Biomedical Research Council of Singapore, the Wellcome Trust, the Biotechnology and Biological Sciences Research Council UK and the American Asthma Foundation.
Author information
Authors and Affiliations
Contributions
P.P., M.A.M., H.K.T., L.A.-R. and J.R. did experiments; S.M.M. supplied reagents; A.J.M. conceived of the study; A.J.M., M.M.H., S.P. and W.H. planned the experiments, supervised the study and wrote the paper; and all authors analyzed data.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–5 (PDF 263 kb)
Rights and permissions
About this article
Cite this article
Puneet, P., McGrath, M., Tay, H. et al. The helminth product ES-62 protects against septic shock via Toll-like receptor 4–dependent autophagosomal degradation of the adaptor MyD88. Nat Immunol 12, 344–351 (2011). https://doi.org/10.1038/ni.2004
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.2004
This article is cited by
-
Regulation of human THP-1 macrophage polarization by Trichinella spiralis
Parasitology Research (2021)
-
Therapeutic effect of Schistosoma japonicum cystatin on bacterial sepsis in mice
Parasites & Vectors (2017)
-
Role of p62 in the suppression of inflammatory cytokine production by adiponectin in macrophages: Involvement of autophagy and p21/Nrf2 axis
Scientific Reports (2017)
-
Trichuris suis soluble products induce Rab7b expression and limit TLR4 responses in human dendritic cells
Genes & Immunity (2015)
-
Role of the TLR4 pathway in blood-spinal cord barrier dysfunction during the bimodal stage after ischemia/reperfusion injury in rats
Journal of Neuroinflammation (2014)