Abstract
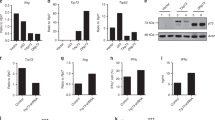
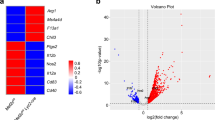
Polymorphisms in the gene encoding the transcription factor IRF5 that lead to higher mRNA expression are associated with many autoimmune diseases. Here we show that IRF5 expression in macrophages was reversibly induced by inflammatory stimuli and contributed to the plasticity of macrophage polarization. High expression of IRF5 was characteristic of M1 macrophages, in which it directly activated transcription of the genes encoding interleukin 12 subunit p40 (IL-12p40), IL-12p35 and IL-23p19 and repressed the gene encoding IL-10. Consequently, those macrophages set up the environment for a potent T helper type 1 (TH1)-TH17 response. Global gene expression analysis demonstrated that exogenous IRF5 upregulated or downregulated expression of established phenotypic markers of M1 or M2 macrophages, respectively. Our data suggest a critical role for IRF5 in M1 macrophage polarization and define a previously unknown function for IRF5 as a transcriptional repressor.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Gordon, S. & Taylor, P.R. Monocyte and macrophage heterogeneity. Nat. Rev. Immunol. 5, 953–964 (2005).
Mosser, D.M. & Edwards, J.P. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 8, 958–969 (2008).
Gordon, S. Alternative activation of macrophages. Nat. Rev. Immunol. 3, 23–35 (2003).
Romagnani, P., Annunziato, F., Piccinni, M.P., Maggi, E. & Romagnani, S. TH1/TH2 cells, their associated molecules and role in pathophysiology. Eur. Cytokine Netw. 11, 510–511 (2000).
Martinez, F.O., Sica, A., Mantovani, A. & Locati, M. Macrophage activation and polarization. Front. Biosci. 13, 453–461 (2008).
Korn, T., Bettelli, E., Oukka, M. & Kuchroo, V.K. IL-17 and TH17 Cells. Annu. Rev. Immunol. 27, 485–517 (2009).
Tamura, T. et al. IFN regulatory factor-4 and -8 govern dendritic cell subset development and their functional diversity. J. Immunol. 174, 2573–2581 (2005).
Porta, C. et al. Tolerance and M2 (alternative) macrophage polarization are related processes orchestrated by p50 nuclear factor κB. Proc. Natl. Acad. Sci. USA 106, 14978–14983 (2009).
Ruffell, D. et al. A CREB-C/EBPβ cascade induces M2 macrophage-specific gene expression and promotes muscle injury repair. Proc. Natl. Acad. Sci. USA 106, 17475–17480 (2009).
Satoh, T. et al. The Jmjd3-Irf4 axis regulates M2 macrophage polarization and host responses against helminth infection. Nat. Immunol. 11, 936–944 (2010).
Ouyang, X. et al. Cooperation between MyD88 and TRIF pathways in TLR synergy via IRF5 activation. Biochem. Biophys. Res. Commun. 354, 1045–1051 (2007).
Takaoka, A. et al. Integral role of IRF-5 in the gene induction programme activated by Toll-like receptors. Nature 434, 243–249 (2005).
Mancl, M.E. et al. Two discrete promoters regulate the alternatively spliced human interferon regulatory factor-5 isoforms. Multiple isoforms with distinct cell type-specific expression, localization, regulation, and function. J. Biol. Chem. 280, 21078–21090 (2005).
Dideberg, V. et al. An insertion-deletion polymorphism in the interferon regulatory factor 5 (IRF5) gene confers risk of inflammatory bowel diseases. Hum. Mol. Genet. 16, 3008–3016 (2007).
Dieguez-Gonzalez, R. et al. Association of interferon regulatory factor 5 haplotypes, similar to that found in systemic lupus erythematosus, in a large subgroup of patients with rheumatoid arthritis. Arthritis Rheum. 58, 1264–1274 (2008).
Graham, R.R. et al. A common haplotype of interferon regulatory factor 5 (IRF5) regulates splicing and expression and is associated with increased risk of systemic lupus erythematosus. Nat. Genet. 38, 550–555 (2006).
Kristjansdottir, G. et al. Interferon regulatory factor 5 (IRF5) gene variants are associated with multiple sclerosis in three distinct populations. J. Med. Genet. 45, 362–369 (2008).
Miceli-Richard, C. et al. Association of an IRF5 gene functional polymorphism with Sjogren's syndrome. Arthritis Rheum. 56, 3989–3994 (2007).
Fleetwood, A.J., Lawrence, T., Hamilton, J.A. & Cook, A.D. Granulocyte-macrophage colony-stimulating factor (CSF) and macrophage CSF-dependent macrophage phenotypes display differences in cytokine profiles and transcription factor activities: implications for CSF blockade in inflammation. J. Immunol. 178, 5245–5252 (2007).
Hoeve, M.A. et al. Divergent effects of IL-12 and IL-23 on the production of IL-17 by human T cells. Eur. J. Immunol. 36, 661–670 (2006).
Verreck, F.A., de Boer, T., Langenberg, D.M., van der Zanden, L. & Ottenhoff, T.H. Phenotypic and functional profiling of human proinflammatory type-1 and anti-inflammatory type-2 macrophages in response to microbial antigens and IFN-γ- and CD40L-mediated costimulation. J. Leukoc. Biol. 79, 285–293 (2006).
Krausgruber, T. et al. IRF5 is required for late-phase TNF secretion by human dendritic cells. Blood 115, 4421–4430 (2010).
Hammer, M. et al. Dual specificity phosphatase 1 (DUSP1) regulates a subset of LPS-induced genes and protects mice from lethal endotoxin shock. J. Exp. Med. 203, 15–20 (2006).
Fleetwood, A.J., Dinh, H., Cook, A.D., Hertzog, P.J. & Hamilton, J.A. GM-CSF- and M-CSF-dependent macrophage phenotypes display differential dependence on type I interferon signaling. J. Leukoc. Biol. 86, 411–421 (2009).
Ahern, P.P. et al. Interleukin-23 drives intestinal inflammation through direct activity on T cells. Immunity 33, 279–288 (2010).
Nistala, K. et al. TH17 plasticity in human autoimmune arthritis is driven by the inflammatory environment. Proc. Natl. Acad. Sci. USA 107, 14751–14756 (2010).
Martinez, F.O., Gordon, S., Locati, M. & Mantovani, A. Transcriptional profiling of the human monocyte-to-macrophage differentiation and polarization: new molecules and patterns of gene expression. J. Immunol. 177, 7303–7311 (2006).
Ziegler-Heitbrock, L. et al. IFN-α induces the human IL-10 gene by recruiting both IFN regulatory factor 1 and Stat3. J. Immunol. 171, 285–290 (2003).
Hamilton, J.A. Colony-stimulating factors in inflammation and autoimmunity. Nat. Rev. Immunol. 8, 533–544 (2008).
Medzhitov, R. & Horng, T. Transcriptional control of the inflammatory response. Nat. Rev. Immunol. 9, 692–703 (2009).
Ghisletti, S. et al. Identification and characterization of enhancers controlling the inflammatory gene expression program in macrophages. Immunity 32, 317–328 (2010).
Negishi, H. et al. Negative regulation of Toll-like-receptor signaling by IRF-4. Proc. Natl. Acad. Sci. USA 102, 15989–15994 (2005).
El Chartouni, C., Schwarzfischer, L. & Rehli, M. Interleukin-4 induced interferon regulatory factor (Irf) 4 participates in the regulation of alternative macrophage priming. Immunobiology 215, 821–825 (2010).
Sanjabi, S., Hoffmann, A., Liou, H.C., Baltimore, D. & Smale, S.T. Selective requirement for c-Rel during IL-12 P40 gene induction in macrophages. Proc. Natl. Acad. Sci. USA 97, 12705–12710 (2000).
Mise-Omata, S. et al. A proximal κB site in the IL-23 p19 promoter is responsible for RelA- and c-Rel-dependent transcription. J. Immunol. 179, 6596–6603 (2007).
Saraiva, M. & O'Garra, A. The regulation of IL-10 production by immune cells. Nat. Rev. Immunol. 10, 170–181 (2010).
Fiorentino, D.F., Zlotnik, A., Mosmann, T.R., Howard, M. & O'Garra, A. IL-10 inhibits cytokine production by activated macrophages. J. Immunol. 147, 3815–3822 (1991).
Wing, K. & Sakaguchi, S. Regulatory T cells exert checks and balances on self tolerance and autoimmunity. Nat. Immunol. 11, 7–13 (2010).
Mosser, D.M. & Zhang, X. Interleukin-10: new perspectives on an old cytokine. Immunol. Rev. 226, 205–218 (2008).
Schneemann, M. & Schoeden, G. Macrophage biology and immunology: man is not a mouse. J. Leukoc. Biol. 81, 579 (2007).
Ponting, C.P. The functional repertoires of metazoan genomes. Nat. Rev. Genet. 9, 689–698 (2008).
Oppmann, B. et al. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity 13, 715–725 (2000).
Romagnani, S., Maggi, E., Liotta, F., Cosmi, L. & Annunziato, F. Properties and origin of human TH17 cells. Mol. Immunol. 47, 3–7 (2009).
Murphy, C.A. et al. Divergent pro- and antiinflammatory roles for IL-23 and IL-12 in joint autoimmune inflammation. J. Exp. Med. 198, 1951–1957 (2003).
Yen, D. et al. IL-23 is essential for T cell-mediated colitis and promotes inflammation via IL-17 and IL-6. J. Clin. Invest. 116, 1310–1316 (2006).
Shen, H. et al. Gender-dependent expression of murine Irf5 gene: implications for sex bias in autoimmunity. J Mol Cell Biol 2, 284–290 (2010).
Campbell, I.K. et al. Protection from collagen-induced arthritis in granulocyte-macrophage colony-stimulating factor-deficient mice. J. Immunol. 161, 3639–3644 (1998).
Cook, A.D., Braine, E.L., Campbell, I.K., Rich, M.J. & Hamilton, J.A. Blockade of collagen-induced arthritis post-onset by antibody to granulocyte-macrophage colony-stimulating factor (GM-CSF): requirement for GM-CSF in the effector phase of disease. Arthritis Res. 3, 293–298 (2001).
Lacaze, P. et al. Combined genome-wide expression profiling and targeted RNA interference in primary mouse macrophages reveals perturbation of transcriptional networks associated with interferon signalling. BMC Genomics 10, 372 (2009).
Liu, J., Cao, S., Herman, L.M. & Ma, X. Differential regulation of interleukin (IL)-12 p35 and p40 gene expression and interferon (IFN)-γ-primed IL-12 production by IFN regulatory factor 1. J. Exp. Med. 198, 1265–1276 (2003).
Acknowledgements
We thank M. Cavanach for assistance with cell-characterization experiments; D. Barban for microarray hybridizations; F.G. Goh, D.G. Saliba and S. Thomson for advice and suggestions on RNA-mediated interference and chromatin immunoprecipitation; X. Ma (Cornell University) for luciferase constructs driven by the IL12A promoter; and C. Monaco and M.E. Goddard for support with animal experiments. Supported by the Medical Research Council (82189 to I.A.U.), the European Community Seventh Framework Programme FP7/2007-2013 (222008) and Arthritis Research UK.
Author information
Authors and Affiliations
Contributions
T.K., T.S., K.B. and S.A. did research; T.K., H.L., N.S. and I.A.U. designed research and analyzed data; and T.K., M.F., T.H. and I.A.U. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–7, Supplementary Tables 1–2 and Supplementary Methods (PDF 3056 kb)
Rights and permissions
About this article
Cite this article
Krausgruber, T., Blazek, K., Smallie, T. et al. IRF5 promotes inflammatory macrophage polarization and TH1-TH17 responses. Nat Immunol 12, 231–238 (2011). https://doi.org/10.1038/ni.1990
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.1990
This article is cited by
-
Peroxiredoxin 3 has a crucial role in the macrophage polarization by regulating mitochondrial homeostasis
Respiratory Research (2024)
-
Longitudinal variation of serum PCSK9 in ulcerative colitis: association with disease activity, T helper 1/2/17 cells, and clinical response of tumor necrosis factor inhibitor
Irish Journal of Medical Science (1971 -) (2024)
-
Exogenous IL-25 ameliorates airway neutrophilia via suppressing macrophage M1 polarization and the expression of IL-12 and IL-23 in asthma
Respiratory Research (2023)
-
Transcriptional regulation on effector T cells in the pathogenesis of psoriasis
European Journal of Medical Research (2023)
-
IRAK4 inhibition: an effective strategy for immunomodulating peri-implant osseointegration via reciprocally-shifted polarization in the monocyte-macrophage lineage cells
BMC Oral Health (2023)