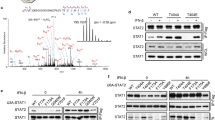
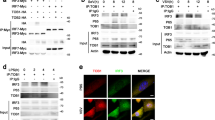
Abstract
Viral infection induces type I interferons (IFN-α and IFN-β) that recruit unexposed cells in a self-amplifying response. We report that the transcription factor MafB thwarts auto-amplification by a metastable switch activity. MafB acted as a weak positive basal regulator of transcription at the IFNB1 promoter through activity at transcription factor AP-1–like sites. Interferon elicitors recruited the transcription factor IRF3 to the promoter, whereupon MafB acted as a transcriptional antagonist, impairing the interaction of coactivators with IRF3. Mathematical modeling supported the view that prepositioning of MafB on the promoter allows the system to respond rapidly to fluctuations in IRF3 activity. Higher expression of MafB in human pancreatic islet beta cells might increase cellular vulnerability to viral infections associated with the etiology of type 1 diabetes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Kawai, T. & Akira, S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat. Immunol. 11, 373–384 (2010).
Yoneyama, M. & Fujita, T. RNA recognition and signal transduction by RIG-I-like receptors. Immunol. Rev. 227, 54–65 (2009).
Motohashi, H., O'Connor, T., Katsuoka, F., Engel, J.D. & Yamamoto, M. Integration and diversity of the regulatory network composed of Maf and CNC families of transcription factors. Gene 294, 1–12 (2002).
Eychene, A., Rocques, N. & Pouponnot, C. A new MAFia in cancer. Nat. Rev. Cancer 8, 683–693 (2008).
Wang, P.W. et al. Human KRML (MAFB): cDNA cloning, genomic structure, and evaluation as a candidate tumor suppressor gene in myeloid leukemias. Genomics 59, 275–281 (1999).
Kelly, L.M., Englmeier, U., Lafon, I., Sieweke, M.H. & Graf, T. MafB is an inducer of monocytic differentiation. EMBO J. 19, 1987–1997 (2000).
Artner, I. et al. MafB: an activator of the glucagon gene expressed in developing islet alpha- and beta-cells. Diabetes 55, 297–304 (2006).
Nishimura, W. et al. A switch from MafB to MafA expression accompanies differentiation to pancreatic beta-cells. Dev. Biol. 293, 526–539 (2006).
Yoneyama, M. et al. The RNA helicase RIG-I has an essential function in double-stranded RNA–induced innate antiviral responses. Nat. Immunol. 5, 730–737 (2004).
Kato, H. et al. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature 441, 101–105 (2006).
Maniatis, T. et al. Structure and function of the interferon-β enhanceosome. Cold Spring Harb. Symp. Quant. Biol. 63, 609–620 (1998).
Escalante, C.R., Nistal-Villan, E., Shen, L., Garcia-Sastre, A. & Aggarwal, A.K. Structure of IRF-3 bound to the PRDIII-I regulatory element of the human interferon-β enhancer. Mol. Cell 26, 703–716 (2007).
Moriguchi, T. et al. MafB is essential for renal development and F4/80 expression in macrophages. Mol. Cell. Biol. 26, 5715–5727 (2006).
Fitzgerald, K.A. et al. IKKɛ and TBK1 are essential components of the IRF3 signaling pathway. Nat. Immunol. 4, 491–496 (2003).
Sharma, S. et al. Triggering the interferon antiviral response through an IKK-related pathway. Science 300, 1148–1151 (2003).
Hiscott, J. Triggering the innate antiviral response through IRF-3 activation. J. Biol. Chem. 282, 15325–15329 (2007).
Tamura, T., Yanai, H., Savitsky, D. & Taniguchi, T. The IRF family transcription factors in immunity and oncogenesis. Annu. Rev. Immunol. 26, 535–584 (2008).
Lin, R., Heylbroeck, C., Pitha, P.M. & Hiscott, J. Virus-dependent phosphorylation of the IRF-3 transcription factor regulates nuclear translocation, transactivation potential, and proteasome-mediated degradation. Mol. Cell. Biol. 18, 2986–2996 (1998).
Lin, R., Genin, P., Mamane, Y. & Hiscott, J. Selective DNA binding and association with the CREB binding protein coactivator contribute to differential activation of α/β interferon genes by interferon regulatory factors 3 and 7. Mol. Cell. Biol. 20, 6342–6353 (2000).
Wathelet, M.G. et al. Virus infection induces the assembly of coordinately activated transcription factors on the IFN-β enhancer in vivo. Mol. Cell 1, 507–518 (1998).
Yoneyama, M. et al. Direct triggering of the type I interferon system by virus infection: activation of a transcription factor complex containing IRF-3 and CBP/p300. EMBO J. 17, 1087–1095 (1998).
Kumar, K.P., McBride, K.M., Weaver, B.K., Dingwall, C. & Reich, N.C. Regulated nuclear-cytoplasmic localization of interferon regulatory factor 3, a subunit of double-stranded RNA-activated factor 1. Mol. Cell. Biol. 20, 4159–4168 (2000).
Cordes, S.P. & Barsh, G.S. The mouse segmentation gene kr encodes a novel basic domain-leucine zipper transcription factor. Cell 79, 1025–1034 (1994).
Sieweke, M.H., Tekotte, H., Frampton, J. & Graf, T. MafB is an interaction partner and repressor of Ets-1 that inhibits erythroid differentiation. Cell 85, 49–60 (1996).
Mizukami, J. & Taniguchi, T. The antidiabetic agent thiazolidinedione stimulates the interaction between PPARγ and CBP. Biochem. Biophys. Res. Commun. 240, 61–64 (1997).
Tillmanns, S. et al. SUMO modification regulates MafB-driven macrophage differentiation by enabling Myb-dependent transcriptional repression. Mol. Cell. Biol. 27, 5554–5564 (2007).
Napolitani, G., Rinaldi, A., Bertoni, F., Sallusto, F. & Lanzavecchia, A. Selected Toll-like receptor agonist combinations synergistically trigger a T helper type 1–polarizing program in dendritic cells. Nat. Immunol. 6, 769–776 (2005).
Stetson, D.B. & Medzhitov, R. Recognition of cytosolic DNA activates an IRF3-dependent innate immune response. Immunity 24, 93–103 (2006).
Pruett, S.B., Schwab, C., Zheng, Q. & Fan, R. Suppression of innate immunity by acute ethanol administration: a global perspective and a new mechanism beginning with inhibition of signaling through TLR3. J. Immunol. 173, 2715–2724 (2004).
Hammer, M. et al. Dual specificity phosphatase 1 (DUSP1) regulates a subset of LPS-induced genes and protects mice from lethal endotoxin shock. J. Exp. Med. 203, 15–20 (2006).
Cao, W. et al. Toll-like receptor-mediated induction of type I interferon in plasmacytoid dendritic cells requires the rapamycin-sensitive PI(3)K-mTOR-p70S6K pathway. Nat. Immunol. 9, 1157–1164 (2008).
Ylipaasto, P. et al. Global profiling of coxsackievirus- and cytokine-induced gene expression in human pancreatic islets. Diabetologia 48, 1510–1522 (2005).
Gaither, L.A. et al. Multiple cyclophilins involved in different cellular pathways mediate HCV replication. Virology 397, 43–55 (2010).
Hyoty, H. & Taylor, K.W. The role of viruses in human diabetes. Diabetologia 45, 1353–1361 (2002).
Ylipaasto, P. et al. Enterovirus infection in human pancreatic islet cells, islet tropism in vivo and receptor involvement in cultured islet beta cells. Diabetologia 47, 225–239 (2004).
Foulis, A.K. et al. A search for the presence of the enteroviral capsid protein VP1 in pancreases of patients with type 1 (insulin-dependent) diabetes and pancreases and hearts of infants who died of coxsackieviral myocarditis. Diabetologia 33, 290–298 (1990).
Maffei, A. et al. Identification of tissue-restricted transcripts in human islets. Endocrinology 145, 4513–4521 (2004).
Marselli, L. et al. Gene expression of purified beta-cell tissue obtained from human pancreas with laser capture microdissection. J. Clin. Endocrinol. Metab. 93, 1046–1053 (2008).
See, D.M. & Tilles, J.G. Pathogenesis of virus-induced diabetes in mice. J. Infect. Dis. 171, 1131–1138 (1995).
Chehadeh, W. et al. Persistent infection of human pancreatic islets by coxsackievirus B is associated with α interferon synthesis in beta cells. J. Virol. 74, 10153–10164 (2000).
Serreze, D.V., Prochazka, M., Reifsnyder, P.C., Bridgett, M.M. & Leiter, E.H. Use of recombinant congenic and congenic strains of NOD mice to identify a new insulin-dependent diabetes resistance gene. J. Exp. Med. 180, 1553–1558 (1994).
Drescher, K.M., Kono, K., Bopegamage, S., Carson, S.D. & Tracy, S. Coxsackievirus B3 infection and type 1 diabetes development in NOD mice: insulitis determines susceptibility of pancreatic islets to virus infection. Virology 329, 381–394 (2004).
Carter, J.D., Dula, S.B., Corbin, K.L., Wu, R. & Nunemaker, C.S. A Practical guide to rodent islet isolation and assessment. Biol. Proced. Online 11, 3–31 (2009).
Senger, K. et al. Gene repression by coactivator repulsion. Mol. Cell 6, 931–937 (2000).
Saitoh, T. et al. Negative regulation of interferon-regulatory factor 3-dependent innate antiviral response by the prolyl isomerase Pin1. Nat. Immunol. 7, 598–605 (2006).
Revollo, J.R. & Cidlowski, J.A. Mechanisms generating diversity in glucocorticoid receptor signaling. Ann. NY Acad. Sci. 1179, 167–178 (2009).
Pouponnot, C. et al. Cell context reveals a dual role for Maf in oncogenesis. Oncogene 25, 1299–1310 (2006).
Whelan, S.P., Ball, L.A., Barr, J.N. & Wertz, G.T. Efficient recovery of infectious vesicular stomatitis virus entirely from cDNA clones. Proc. Natl. Acad. Sci. USA 92, 8388–8392 (1995).
Iwamura, T. et al. Induction of IRF-3/-7 kinase and NF-κB in response to double-stranded RNA and virus infection: common and unique pathways. Genes Cells 6, 375–388 (2001).
Kim, H. & Perelson, A.S. Viral and latent reservoir persistence in HIV-1-infected patients on therapy. PLOS Comput. Biol. 2, e135 (2006).
Acknowledgements
We thank J. Darga for assistance with automated assays and high-throughput screens; R. Niedra, N. Lu and W. Zhou for experimental help; the Seed and Xavier groups for sharing reagents and information; S. Takahashi and M. Hamada (University of Tsukuba) and D. Engel (University of Michigan) for Mafb-deficient MEFs and discussions; S. Whelan (Harvard Medical School) for VSV-Luc; T. Fujita (Kyoto University) for the CBP construct; J.-i. Miyazaki (Osaka University) and D.F. Steiner (University of Chicago) for MIN6 cells; C. Vanderburg for assistance with laser-capture microdissection; and M. Wathelet, R. Lin, S. Han and H. Lee for discussions.
Author information
Authors and Affiliations
Contributions
H.K. designed and did experiments and wrote the manuscript; B.S. designed and supervised research and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–9, Supplementary Tables 1–3 and Supplementary Methods (PDF 3615 kb)
Rights and permissions
About this article
Cite this article
Kim, H., Seed, B. The transcription factor MafB antagonizes antiviral responses by blocking recruitment of coactivators to the transcription factor IRF3. Nat Immunol 11, 743–750 (2010). https://doi.org/10.1038/ni.1897
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.1897
This article is cited by
-
Macrophage re-programming by JAK inhibitors relies on MAFB
Cellular and Molecular Life Sciences (2024)
-
What’s happening where when SARS-CoV-2 infects: are TLR7 and MAFB sufficient to explain patient vulnerability?
Immunity & Ageing (2022)
-
Kidney dendritic cells: fundamental biology and functional roles in health and disease
Nature Reviews Nephrology (2020)
-
Loss of MafA and MafB expression promotes islet inflammation
Scientific Reports (2019)
-
Dysregulated heme oxygenase-1low M2-like macrophages augment lupus nephritis via Bach1 induced by type I interferons
Arthritis Research & Therapy (2018)