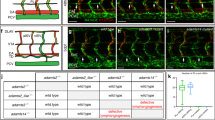
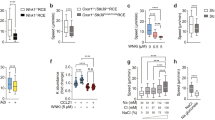
Abstract
The recirculation of leukocytes is essential for proper immune responses. However, the molecular mechanisms that regulate the entry of leukocytes into the lymphatics remain unclear. Here we show that plexin-A1, a principal receptor component for class III and class VI semaphorins, was crucially involved in the entry of dendritic cells (DCs) into the lymphatics. Additionally, we show that the semaphorin Sema3A, but not Sema6C or Sema6D, was required for DC transmigration and that Sema3A produced by the lymphatics promoted actomyosin contraction at the trailing edge of migrating DCs. Our findings not only demonstrate that semaphorin signals are involved in DC trafficking but also identify a previously unknown mechanism that induces actomyosin contraction as these cells pass through narrow gaps.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Steinman, R.M. & Banchereau, J. Taking dendritic cells into medicine. Nature 449, 419–426 (2007).
Randolph, G.J., Angeli, V. & Swartz, M.A. Dendritic-cell trafficking to lymph nodes through lymphatic vessels. Nat. Rev. Immunol. 5, 617–628 (2005).
Alvarez, D., Vollmann, E.H. & von Andrian, U.H. Mechanisms and consequences of dendritic cell migration. Immunity 29, 325–342 (2008).
Forster, R. et al. CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell 99, 23–33 (1999).
MartIn-Fontecha, A. et al. Regulation of dendritic cell migration to the draining lymph node: impact on T lymphocyte traffic and priming. J. Exp. Med. 198, 615–621 (2003).
Ohl, L. et al. CCR7 governs skin dendritic cell migration under inflammatory and steady-state conditions. Immunity 21, 279–288 (2004).
Johnson, L.A. et al. An inflammation-induced mechanism for leukocyte transmigration across lymphatic vessel endothelium. J. Exp. Med. 203, 2763–2777 (2006).
Kabashima, K. et al. CXCL12-CXCR4 engagement is required for migration of cutaneous dendritic cells. Am. J. Pathol. 171, 1249–1257 (2007).
Stoitzner, P., Pfaller, K., Stossel, H. & Romani, N. A close-up view of migrating Langerhans cells in the skin. J. Invest. Dermatol. 118, 117–125 (2002).
Baluk, P. et al. Functionally specialized junctions between endothelial cells of lymphatic vessels. J. Exp. Med. 204, 2349–2362 (2007).
Lammermann, T. et al. Rapid leukocyte migration by integrin-independent flowing and squeezing. Nature 453, 51–55 (2008).
Kolodkin, A.L., Matthes, D.J. & Goodman, C.S. The semaphorin genes encode a family of transmembrane and secreted growth cone guidance molecules. Cell 75, 1389–1399 (1993).
Toyofuku, T. et al. Dual roles of Sema6D in cardiac morphogenesis through region-specific association of its receptor, Plexin-A1, with off-track and vascular endothelial growth factor receptor type 2. Genes Dev. 18, 435–447 (2004).
Serini, G. et al. Class 3 semaphorins control vascular morphogenesis by inhibiting integrin function. Nature 424, 391–397 (2003).
Neufeld, G. & Kessler, O. The semaphorins: versatile regulators of tumour progression and tumour angiogenesis. Nat. Rev. Cancer 8, 632–645 (2008).
Capparuccia, L. & Tamagnone, L. Semaphorin signaling in cancer cells and in cells of the tumor microenvironment–two sides of a coin. J. Cell Sci. 122, 1723–1736 (2009).
Suzuki, K., Kumanogoh, A. & Kikutani, H. Semaphorins and their receptors in immune cell interactions. Nat. Immunol. 9, 17–23 (2008).
Kumanogoh, A. et al. Identification of CD72 as a lymphocyte receptor for the class IV semaphorin CD100: a novel mechanism for regulating B cell signaling. Immunity 13, 621–631 (2000).
Kumanogoh, A. et al. Nonredundant roles of Sema4A in the immune system: defective T cell priming and Th1/Th2 regulation in Sema4A-deficient mice. Immunity 22, 305–316 (2005).
Takegahara, N. et al. Plexin-A1 and its interaction with DAP12 in immune responses and bone homeostasis. Nat. Cell Biol. 8, 615–622 (2006).
Suzuki, K. et al. Semaphorin 7A initiates T-cell-mediated inflammatory responses through α1β1 integrin. Nature 446, 680–684 (2007).
Kruger, R.P., Aurandt, J. & Guan, K.L. Semaphorins command cells to move. Nat. Rev. Mol. Cell Biol. 6, 789–800 (2005).
Takahashi, T. et al. Plexin-neuropilin-1 complexes form functional semaphorin-3A receptors. Cell 99, 59–69 (1999).
Yoshida, Y., Han, B., Mendelsohn, M. & Jessell, T.M. PlexinA1 signaling directs the segregation of proprioceptive sensory axons in the developing spinal cord. Neuron 52, 775–788 (2006).
Wong, A.W. et al. CIITA-regulated plexin-A1 affects T-cell-dendritic cell interactions. Nat. Immunol. 4, 891–898 (2003).
Barnden, M.J., Allison, J., Heath, W.R. & Carbone, F.R. Defective TCR expression in transgenic mice constructed using cDNA-based α- and β-chain genes under the control of heterologous regulatory elements. Immunol. Cell Biol. 76, 34–40 (1998).
Mempel, T.R., Henrickson, S.E. & Von Andrian, U.H. T-cell priming by dendritic cells in lymph nodes occurs in three distinct phases. Nature 427, 154–159 (2004).
Shortman, K. & Liu, Y.J. Mouse and human dendritic cell subtypes. Nat. Rev. Immunol. 2, 151–161 (2002).
Macatonia, S.E., Knight, S.C., Edwards, A.J., Griffiths, S. & Fryer, P. Localization of antigen on lymph node dendritic cells after exposure to the contact sensitizer fluorescein isothiocyanate. Functional and morphological studies. J. Exp. Med. 166, 1654–1667 (1987).
Angeli, V. et al. B cell-driven lymphangiogenesis in inflamed lymph nodes enhances dendritic cell mobilization. Immunity 24, 203–215 (2006).
Taniguchi, M. et al. Disruption of semaphorin III/D gene causes severe abnormality in peripheral nerve projection. Neuron 19, 519–530 (1997).
Gu, C. et al. Neuropilin-1 conveys semaphorin and VEGF signaling during neural and cardiovascular development. Dev. Cell 5, 45–57 (2003).
Clark, K., Langeslag, M., Figdor, C.G. & van Leeuwen, F.N. Myosin II and mechanotransduction: a balancing act. Trends Cell Biol. 17, 178–186 (2007).
Gallo, G. RhoA-kinase coordinates F-actin organization and myosin II activity during semaphorin-3A-induced axon retraction. J. Cell Sci. 119, 3413–3423 (2006).
Brown, J.A., Wysolmerski, R.B. & Bridgman, P.C. Dorsal root ganglion neurons react to semaphorin 3A application through a biphasic response that requires multiple myosin II isoforms. Mol. Biol. Cell 20, 1167–1179 (2009).
Boulanger, L.M. Immune proteins in brain development and synaptic plasticity. Neuron 64, 93–109 (2009).
Cumberbatch, M. & Kimber, I. Tumour necrosis factor-α is required for accumulation of dendritic cells in draining lymph nodes and for optimal contact sensitization. Immunology 84, 31–35 (1995).
Ratzinger, G. et al. Matrix metalloproteinases 9 and 2 are necessary for the migration of Langerhans cells and dermal dendritic cells from human and murine skin. J. Immunol. 168, 4361–4371 (2002).
Lauffenburger, D.A. & Horwitz, A.F. Cell migration: a physically integrated molecular process. Cell 84, 359–369 (1996).
Chhabra, E.S. & Higgs, H.N. The many faces of actin: matching assembly factors with cellular structures. Nat. Cell Biol. 9, 1110–1121 (2007).
Vicente-Manzanares, M., Ma, X., Adelstein, R.S. & Horwitz, A.R. Non-muscle myosin II takes centre stage in cell adhesion and migration. Nat. Rev. Mol. Cell Biol. 10, 778–790 (2009).
Wu, K.Y. et al. Local translation of RhoA regulates growth cone collapse. Nature 436, 1020–1024 (2005).
Hengst, U. & Jaffrey, S.R. Function and translational regulation of mRNA in developing axons. Semin. Cell Dev. Biol. 18, 209–215 (2007).
Friedl, P. & Weigelin, B. Interstitial leukocyte migration and immune function. Nat. Immunol. 9, 960–969 (2008).
Lammermann, T. & Sixt, M. Mechanical modes of 'amoeboid' cell migration. Curr. Opin. Cell Biol. 21, 636–644 (2009).
Lombardi, M.L., Knecht, D.A., Dembo, M. & Lee, J. Traction force microscopy in Dictyostelium reveals distinct roles for myosin II motor and actin-crosslinking activity in polarized cell movement. J. Cell Sci. 120, 1624–1634 (2007).
Smith, L.A., Aranda-Espinoza, H., Haun, J.B., Dembo, M. & Hammer, D.A. Neutrophil traction stresses are concentrated in the uropod during migration. Biophys. J. 92, L58–L60 (2007).
Toyofuku, T. et al. FARP2 triggers signals for Sema3A-mediated axonal repulsion. Nat. Neurosci. 8, 1712–1719 (2005).
Cavanagh, L.L. et al. Activation of bone marrow–resident memory T cells by circulating, antigen-bearing dendritic cells. Nat. Immunol. 6, 1029–1037 (2005).
Ledgerwood, L.G. et al. The sphingosine 1-phosphate receptor 1 causes tissue retention by inhibiting the entry of peripheral tissue T lymphocytes into afferent lymphatics. Nat. Immunol. 9, 42–53 (2008).
Acknowledgements
We thank D.D. Ginty and A.L. Kolodkin (Howard Hughes Medical Institute) for neuropilin-1-mutant mice; W.R. Heath (University of Melbourne) for OT-II-transgenic mice; and T. Yazawa for technical support. Supported by the Japan Society for the Promotion of Science (Research Fellowships for Young Scientists to H.T.), the US National Institutes of Health (R01NS065048 to Y.Y.), the Ministry of Education, Culture, Sports, Science and Technology of Japan, the Ministry of Health, Labour and Welfare, the Program for Promotion of Fundamental Studies in Health Sciences of the National Institute of Biomedical Innovation (A.K.), the Target Protein Research Program of the Japan Science and Technology Agency (T.T. and A.K.), the Uehara Memorial Foundation (A.K.) and the Takeda Scientific Foundation (T.T., N.T. and A.K.).
Author information
Authors and Affiliations
Contributions
A.K. and H.T. designed the study and wrote the manuscript; H.T. did most of the experiments and analyzed the data with Y. Nakagawa, T.O., M.M., S.K. and S.N.; N.T. produced Sema6d−/− mice, recombinant Sema6D protein and antibody to Sema6D (anti-Sema6D); M. Tomura did two-photon microscopic experiments; M. Tanaguchi produced Sema3a−/− mice; R.H.F., H.R. and M.T.-L. produced Sema6c−/− mice; Y.Y. produced anti-plexin-A1; T. Tsujimura did histological analyses; and Y. Nakatsuji, I.K., T. Toyofuku and H.K. provided collaborative suggestions.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–7 and Supplementary Methods (PDF 1548 kb)
Supplementary Video 1
Normal to sense direction in response to chemokines in plexin-A1−/− DCs. DCs from wild-type or plexin-A1−/− mice were allowed to adhere to fibronectin-coated cover-slips and placed in a CCL21 gradient in a Zigmond chamber. DC trafficking was recorded every 30 sec by a confocal time-lapse video microscope. (MOV 1612 kb)
Supplementary Video 2
Plexin-A1−/− DCs exhibit impaired transmigration across a lymphatic EC-monolayer. BMDCs derived from wild-type or plexin-A1−/− mice were added to lymphatic EC monolayers, and interactions between DCs and the lymphatic ECs were recorded every 30 sec by a time-lapse video microscope. The yellow dotted lines show the cellular junctions of the ECs. White arrows indicate DCs that were contacting the lymphatic ECs. (MOV 4486 kb)
Supplementary Video 3
Impaired transmigration in plexin-A1−/− DCs. CFSE-labeled DCs derived from wild-type or plexin-A1−/− mice were added to lymphatic EC monolayers, incubated for 45 min, fixed, and then stained with Alexa 546-conjugated phalloidin. Confocal microscope images were obtained with an optical section separation (Z-interval) of 0.22 μm. Twelve Z-stack images were reconstituted into a 3Dimage using Imaris 3D software. Wild-type DCs penetrated from the apical to basal sides, but plexin-A1−/− DCs could not reach the basal side. (MOV 979 kb)
Supplementary Video 4
Sema3A acts on the rear side of migrating DCs. Two-dimensional DC chemotaxis assays using EZ-TAXIScan were performed, in which recombinant Sema3A or human IgG was applied to the opposite side of CCL21. DC migration was recorded every 30 sec by a time-lapse video microscope. (MOV 977 kb)
Supplementary Video 5
Plexin-A1 localizes to the back of migrating DCs. Plexin-A1-EGFP fusion protein-expressed BMDCs treated with LPS were suspended in type I collagen (3 mg/ml) containing 2% FCS and then placed on one side of the Zigmond chamber to cover the stage with gel. After gel was polymerized, CCL21was added to the other side. DC locomotion was examined at 1-min intervals by a confocal time-lapse video microscope. (MOV 191 kb)
Supplementary Video 6
Sema3A enhances the velocity of DC migration in 3D-collagen matrices. BMDCs treated with LPS for 12 h were suspended in type I collagen (3 mg/ml) containing 2% FCS with either a human IgG or Sema3A-Fc and then placed on one side of the Zigmond chamber to cover the stage with gel. The cells were incubated at 37C for 30 min to polymerize the matrix, and then RPMI containing 0.5% BSA with CCL21 (5 μg/ml) was added to the other chamber. After a 20-min incubation, DC locomotion was examined at 1-min intervals by a confocal time-lapse video microscope. (MOV 1449 kb)
Rights and permissions
About this article
Cite this article
Takamatsu, H., Takegahara, N., Nakagawa, Y. et al. Semaphorins guide the entry of dendritic cells into the lymphatics by activating myosin II. Nat Immunol 11, 594–600 (2010). https://doi.org/10.1038/ni.1885
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.1885
This article is cited by
-
Semaphorin 3A: A potential target for prevention and treatment of nickel allergy
Communications Biology (2022)
-
Semaphorin-3A: a promising therapeutic tool in allergic rhinitis
Immunologic Research (2022)
-
Semaphorin3A increases M1-like microglia and retinal ganglion cell apoptosis after optic nerve injury
Cell & Bioscience (2021)
-
Plexin-B2 facilitates glioblastoma infiltration by modulating cell biomechanics
Communications Biology (2021)
-
The lysosomal Ragulator complex plays an essential role in leukocyte trafficking by activating myosin II
Nature Communications (2021)