Abstract
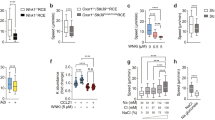
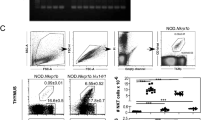
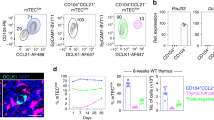
Thymocytes are highly motile cells that migrate under the influence of chemokines in distinct thymic compartments as they mature. The motility of thymocytes is tightly regulated; however, the molecular mechanisms that control thymocyte motility are not well understood. Here we report that G protein–coupled receptor kinase-interactor 2 (GIT2) was required for efficient positive selection. Notably, Git2−/− double-positive thymocytes showed greater activation of the small GTPase Rac, actin polymerization and migration toward the chemokines CXCL12 (SDF-1) and CCL25 in vitro. By two-photon laser-scanning microscopy, we found that the scanning activity of Git2−/− thymocytes was compromised in the thymic cortex, which suggests GIT2 has a key role in regulating the chemokine-mediated motility of double-positive thymocytes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Ladi, E., Yin, X., Chtanova, T. & Robey, E.A. Thymic microenvironments for T cell differentiation and selection. Nat. Immunol. 7, 338–343 (2006).
Bousso, P., Bhakta, N.R., Lewis, R.S. & Robey, E. Dynamics of thymocyte-stromal cell interactions visualized by two-photon microscopy. Science 296, 1876–1880 (2002).
Witt, C.M., Raychaudhuri, S., Schaefer, B., Chakraborty, A.K. & Robey, E.A. Directed migration of positively selected thymocytes visualized in real time. PLoS Biol. 3, e160 (2005).
Bhakta, N.R., Oh, D.Y. & Lewis, R.S. Calcium oscillations regulate thymocyte motility during positive selection in the three-dimensional thymic environment. Nat. Immunol. 6, 143–151 (2005).
Ladi, E. et al. Thymocyte-dendritic cell interactions near sources of CCR7 ligands in the thymic cortex. J. Immunol. 181, 7014–7023 (2008).
Suzuki, G. et al. Pertussis toxin-sensitive signal controls the trafficking of thymocytes across the corticomedullary junction in the thymus. J. Immunol. 162, 5981–5985 (1999).
Zaitseva, M.B. et al. CXCR4 and CCR5 on human thymocytes: biological function and role in HIV-1 infection. J. Immunol. 161, 3103–3113 (1998).
Wurbel, M.A. et al. The chemokine TECK is expressed by thymic and intestinal epithelial cells and attracts double- and single-positive thymocytes expressing the TECK receptor CCR9. Eur. J. Immunol. 30, 262–271 (2000).
Misslitz, A. et al. Thymic T cell development and progenitor localization depend on CCR7. J. Exp. Med. 200, 481–491 (2004).
Uehara, S., Song, K., Farber, J.M. & Love, P.E. Characterization of CCR9 expression and CCL25/thymus-expressed chemokine responsiveness during T cell development: CD3highCD69+ thymocytes and gammadeltaTCR+ thymocytes preferentially respond to CCL25. J. Immunol. 168, 134–142 (2002).
Choi, Y.I. et al. PlexinD1 glycoprotein controls migration of positively selected thymocytes into the medulla. Immunity 29, 888–898 (2008).
Turner, C.E. Paxillin interactions. J. Cell Sci. 113, 4139–4140 (2000).
Frank, S.R. & Hansen, S.H. The PIX-GIT complex: a G protein signaling cassette in control of cell shape. Semin. Cell Dev. Biol. 19, 234–244 (2008).
Randazzo, P.A., Inoue, H. & Bharti, S. Arf GAPs as regulators of the actin cytoskeleton. Biol. Cell 99, 583–600 (2007).
Hoefen, R.J. & Berk, B.C. The multifunctional GIT family of proteins. J. Cell Sci. 119, 1469–1475 (2006).
Sabe, H., Onodera, Y., Mazaki, Y. & Hashimoto, S. ArfGAP family proteins in cell adhesion, migration and tumor invasion. Curr. Opin. Cell Biol. 18, 558–564 (2006).
Vitale, N. et al. GIT proteins, A novel family of phosphatidylinositol 3,4, 5-trisphosphate-stimulated GTPase-activating proteins for ARF6. J. Biol. Chem. 275, 13901–13906 (2000).
Premont, R.T., Claing, A., Vitale, N., Perry, S.J. & Lefkowitz, R.J. The GIT family of ADP-ribosylation factor GTPase-activating proteins. Functional diversity of GIT2 through alternative splicing. J. Biol. Chem. 275, 22373–22380 (2000).
Radhakrishna, H., Al-Awar, O., Khachikian, Z. & Donaldson, J.G. ARF6 requirement for Rac ruffling suggests a role for membrane trafficking in cortical actin rearrangements. J. Cell Sci. 112, 855–866 (1999).
D'Souza-Schorey, C. & Chavrier, P. ARF proteins: roles in membrane traffic and beyond. Nat. Rev. Mol. Cell Biol. 7, 347–358 (2006).
Santy, L.C. & Casanova, J.E. Activation of ARF6 by ARNO stimulates epithelial cell migration through downstream activation of both Rac1 and phospholipase D. J. Cell Biol. 154, 599–610 (2001).
Premont, R.T. et al. beta2-Adrenergic receptor regulation by GIT1, a G protein-coupled receptor kinase-associated ADP ribosylation factor GTPase-activating protein. Proc. Natl. Acad. Sci. USA 95, 14082–14087 (1998).
Bagrodia, S. et al. A tyrosine-phosphorylated protein that binds to an important regulatory region on the cool family of p21-activated kinase-binding proteins. J. Biol. Chem. 274, 22393–22400 (1999).
Turner, C.E. et al. Paxillin LD4 motif binds PAK and PIX through a novel 95-kD ankyrin repeat, ARF-GAP protein: A role in cytoskeletal remodeling. J. Cell Biol. 145, 851–863 (1999).
Frank, S.R., Adelstein, M.R. & Hansen, S.H. GIT2 represses Crk- and Rac1-regulated cell spreading and Cdc42-mediated focal adhesion turnover. EMBO J. 25, 1848–1859 (2006).
Zhang, H., Webb, D.J., Asmussen, H., Niu, S. & Horwitz, A.F.A. GIT1/PIX/Rac/PAK signaling module regulates spine morphogenesis and synapse formation through MLC. J. Neurosci. 25, 3379–3388 (2005).
Mazaki, Y. et al. Neutrophil direction sensing and superoxide production linked by the GTPase-activating protein GIT2. Nat. Immunol. 7, 724–731 (2006).
Nishiya, N., Kiosses, W.B., Han, J. & Ginsberg, M.H. An α4 integrin-paxillin-Arf-GAP complex restricts Rac activation to the leading edge of migrating cells. Nat. Cell Biol. 7, 343–352 (2005).
Ku, G.M., Yablonski, D., Manser, E., Lim, L. & Weiss, A.A. PAK1-PIX-PKL complex is activated by the T-cell receptor independent of Nck, Slp-76 and LAT. EMBO J. 20, 457–465 (2001).
Phee, H., Abraham, R.T. & Weiss, A. Dynamic recruitment of PAK1 to the immunological synapse is mediated by PIX independently of SLP-76 and Vav1. Nat. Immunol. 6, 608–617 (2005).
Schmalzigaug, R., Phee, H., Davidson, C.E., Weiss, A. & Premont, R.T. Differential expression of the ARF GAP genes GIT1 and GIT2 in mouse tissues. J. Histochem. Cytochem. 55, 1039–1048 (2007).
Kisielow, P., Bluthmann, H., Staerz, U.D., Steinmetz, M. & von Boehmer, H. Tolerance in T-cell-receptor transgenic mice involves deletion of nonmature CD4+8+ thymocytes. Nature 333, 742–746 (1988).
Kisielow, P., Teh, H.S., Bluthmann, H. & von Boehmer, H. Positive selection of antigen-specific T cells in thymus by restricting MHC molecules. Nature 335, 730–733 (1988).
Azzam, H.S. et al. CD5 expression is developmentally regulated by T cell receptor (TCR) signals and TCR avidity. J. Exp. Med. 188, 2301–2311 (1998).
Campbell, J.J., Pan, J. & Butcher, E.C. Cutting edge: developmental switches in chemokine responses during T cell maturation. J. Immunol. 163, 2353–2357 (1999).
Hori, Y., Winans, A.M., Huang, C.C., Horrigan, E.M. & Irvine, D.J. Injectable dendritic cell-carrying alginate gels for immunization and immunotherapy. Biomaterials 29, 3671–3682 (2008).
Bromley, S.K., Peterson, D.A., Gunn, M.D. & Dustin, M.L. Cutting edge: hierarchy of chemokine receptor and TCR signals regulating T cell migration and proliferation. J. Immunol. 165, 15–19 (2000).
Trampont, P.C. et al. CXCR4 acts as a costimulator during thymic β-selection. Nat. Immunol. 11, 162–170 (2010).
Kato, S. Thymic microvascular system. Microsc. Res. Tech. 38, 287–299 (1997).
Shiow, L.R. et al. The actin regulator coronin 1A is mutant in a thymic egress-deficient mouse strain and in a patient with severe combined immunodeficiency. Nat. Immunol. 9, 1307–1315 (2008).
Jacobelli, J., Bennett, F.C., Pandurangi, P., Tooley, A.J. & Krummel, M.F. Myosin-IIA and ICAM-1 regulate the interchange between two distinct modes of T cell migration. J. Immunol. 182, 2041–2050 (2009).
Acknowledgements
We thank the Transgenic/Targeted Mutagenesis Core Facility of the University of California San Francisco Comprehensive Cancer Center for creating Git2−/− mice; the Biological Imaging Development Center of the University of California San Francisco for Imaris software support; and E. Manser (Institute of Molecular and Cell Biology, Singapore) for monoclonal and polyclonal antibodies that recognize the α- and β-isoforms of PIX. Supported by the Leukemia and Lymphoma Society (H.P.) and the National Institutes of Health (R01AI064227 to E.R.).
Author information
Authors and Affiliations
Contributions
H.P. designed the study, did experiments, analyzed data and wrote the manuscript; M.M. did experiments; I.D. did the two-photon experiments and analyzed data; Y.W. and D.J.I. supplied alginate beads; E.R. supervised the two-photon experiments and discussed data; and A.W. designed the study, supervised the research and revised the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–8, Table 1 and Methods (PDF 4120 kb)
Supplementary Video 1
Directional migration of wild-type and Git2−/− thymocytes to SDF-1. (AVI 3025 kb)
Supplementary Video 2
Migration of wild-type and Git2−/− thymocytes in intact thymic lobes using two-photon microscopy. (AVI 947 kb)
Supplementary Video 3
Accumulation and movement of Git2−/− thymocyte on small blood vessels. (AVI 1062 kb)
Supplementary Video 4
Migratory behavior of wild-type thymocytes that move away from small blood vessels. (AVI 284 kb)
Rights and permissions
About this article
Cite this article
Phee, H., Dzhagalov, I., Mollenauer, M. et al. Regulation of thymocyte positive selection and motility by GIT2. Nat Immunol 11, 503–511 (2010). https://doi.org/10.1038/ni.1868
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.1868
This article is cited by
-
The focal adhesion-associated proteins DOCK5 and GIT2 comprise a rheostat in control of epithelial invasion
Oncogene (2017)
-
Thymic stromal cell subsets for T cell development
Cellular and Molecular Life Sciences (2016)
-
Mutations in components of antiviral or microbial defense as a basis for breast cancer
Functional & Integrative Genomics (2013)
-
Mst1 regulates integrin-dependent thymocyte trafficking and antigen recognition in the thymus
Nature Communications (2012)
-
How to find your way through the thymus: a practical guide for aspiring T cells
Cellular and Molecular Life Sciences (2012)