Abstract
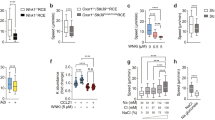
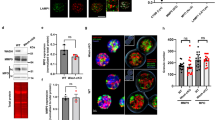
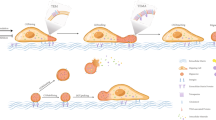
Chemokines and other chemoattractants direct leukocyte migration and are essential for the development and delivery of immune and inflammatory responses. To probe the molecular mechanisms that underlie chemoattractant-guided migration, we did an RNA-mediated interference screen that identified several members of the synaptotagmin family of calcium-sensing vesicle-fusion proteins as mediators of cell migration: SYT7 and SYTL5 were positive regulators of chemotaxis, whereas SYT2 was a negative regulator of chemotaxis. SYT7-deficient leukocytes showed less migration in vitro and in a gout model in vivo. Chemoattractant-induced calcium-dependent lysosomal fusion was impaired in SYT7-deficient neutrophils. In a chemokine gradient, SYT7-deficient lymphocytes accumulated lysosomes in their uropods and had impaired uropod release. Our data identify a molecular pathway required for chemotaxis that links chemoattractant-induced calcium flux to exocytosis and uropod release.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Viola, A. & Luster, A.D. Chemokines and their receptors: drug targets in immunity and inflammation. Annu. Rev. Pharmacol. Toxicol. 48, 171–197 (2008).
Murphy, P.M. The molecular biology of leukocyte chemoattractant receptors. Annu. Rev. Immunol. 12, 593–633 (1994).
Ridley, A.J. et al. Cell migration: integrating signals from front to back. Science 302, 1704–1709 (2003).
Van Haastert, P.J. & Devreotes, P.N. Chemotaxis: signalling the way forward. Nat. Rev. Mol. Cell Biol. 5, 626–634 (2004).
Kehrl, J.H., Hwang, I.Y. & Park, C. Chemoattract receptor signaling and its role in lymphocyte motility and trafficking. Curr. Top. Microbiol. Immunol. 334, 107–127 (2009).
Wu, D., LaRosa, G.J. & Simon, M.I. G protein-coupled signal transduction pathways for interleukin-8. Science 261, 101–103 (1993).
Malchow, D., Bohme, R. & Rahmsdorf, H.J. Regulation of phosphorylation of myosin heavy chain during the chemotactic response of Dictyostelium cells. Eur. J. Biochem. 117, 213–218 (1981).
Liu, G. & Newell, P.C. Regulation of myosin regulatory light chain phosphorylation via cyclic GMP during chemotaxis of Dictyostelium. J. Cell Sci. 107, 1737–1743 (1994).
Pettit, E.J. & Fay, F.S. Cytosolic free calcium and the cytoskeleton in the control of leukocyte chemotaxis. Physiol. Rev. 78, 949–967 (1998).
Brundage, R.A., Fogarty, K.E., Tuft, R.A. & Fay, F.S. Calcium gradients underlying polarization and chemotaxis of eosinophils. Science 254, 703–706 (1991).
Stoyanov, B. et al. Cloning and characterization of a G protein-activated human phosphoinositide-3 kinase. Science 269, 690–693 (1995).
Stephens, L. et al. A novel phosphoinositide 3 kinase activity in myeloid-derived cells is activated by G protein βγ subunits. Cell 77, 83–93 (1994).
Hannigan, M.O., Huang, C.K. & Wu, D.Q. Roles of PI3K in neutrophil function. Curr. Top. Microbiol. Immunol. 282, 165–175 (2004).
Thelen, M. & Stein, J.V. How chemokines invite leukocytes to dance. Nat. Immunol. 9, 953–959 (2008).
Zou, Y.R., Kottmann, A.H., Kuroda, M., Taniuchi, I. & Littman, D.R. Function of the chemokine receptor CXCR4 in haematopoiesis and in cerebellar development. Nature 393, 595–599 (1998).
Ma, Q. et al. Impaired B-lymphopoiesis, myelopoiesis, and derailed cerebellar neuron migration in CXCR4- and SDF-1-deficient mice. Proc. Natl. Acad. Sci. USA 95, 9448–9453 (1998).
Root, D.D., Hacohen, N., Hahn, W.C., Lander, E.S. & Sabatini, D.M. Genome-scale loss-of-function screening with a lentiviral RNAi library. Nat. Methods 3, 715–719 (2006).
Colvin, R.A., Campanella, G.S., Sun, J. & Luster, A.D. Intracellular domains of CXCR3 that mediate CXCL9, CXCL10, and CXCL11 function. J. Biol. Chem. 279, 30219–30227 (2004).
Koh, A. et al. Role of osteopontin in neutrophil function. Immunology 122, 466–475 (2007).
Ticchioni, M. et al. Signaling through ZAP-70 is required for CXCL12-mediated T-cell transendothelial migration. Blood 99, 3111–3118 (2002).
Baram, D. et al. Synaptotagmin II negatively regulates Ca2+-triggered exocytosis of lysosomes in mast cells. J. Exp. Med. 189, 1649–1658 (1999).
Martinez, I. et al. Synaptotagmin VII regulates Ca2+-dependent exocytosis of lysosomes in fibroblasts. J. Cell Biol. 148, 1141–1149 (2000).
Reddy, A., Caler, E.V. & Andrews, N.W. Plasma membrane repair is mediated by Ca2+-regulated exocytosis of lysosomes. Cell 106, 157–169 (2001).
Chakrabarti, S. et al. Impaired membrane resealing and autoimmune myositis in synaptotagmin VII-deficient mice. J. Cell Biol. 162, 543–549 (2003).
Stinchcombe, J.C. et al. Rab27a is required for regulated secretion in cytotoxic T lymphocytes. J. Cell Biol. 152, 825–834 (2001).
Kuroda, T.S., Fukuda, M., Ariga, H. & Mikoshiba, K. Synaptotagmin-like protein 5: a novel Rab27A effector with C-terminal tandem C2 domains. Biochem. Biophys. Res. Commun. 293, 899–906 (2002).
Coppola, T. et al. Direct interaction of the Rab3 effector RIM with Ca2+ channels, SNAP-25, and synaptotagmin. J. Biol. Chem. 276, 32756–32762 (2001).
Geppert, M., Goda, Y., Stevens, C.F. & Sudhof, T.C. The small GTP-binding protein Rab3A regulates a late step in synaptic vesicle fusion. Nature 387, 810–814 (1997).
Zigmond, S.H. Orientation chamber in chemotaxis. Methods Enzymol. 162, 65–72 (1988).
Chen, C.J. et al. MyD88-dependent IL-1 receptor signaling is essential for gouty inflammation stimulated by monosodium urate crystals. J. Clin. Invest. 116, 2262–2271 (2006).
Martin, W.J., Walton, M. & Harper, J. Resident macrophages initiating and driving inflammation in a monosodium urate monohydrate crystal-induced murine peritoneal model of acute gout. Arthritis Rheum. 60, 281–289 (2009).
Terkeltaub, R., Baird, S., Sears, P., Santiago, R. & Boisvert, W. The murine homolog of the interleukin-8 receptor CXCR-2 is essential for the occurrence of neutrophilic inflammation in the air pouch model of acute urate crystal-induced gouty synovitis. Arthritis Rheum. 41, 900–909 (1998).
Manes, S. et al. Mastering time and space: immune cell polarization and chemotaxis. Semin. Immunol. 17, 77–86 (2005).
Izumi, T. Physiological roles of Rab27 effectors in regulated exocytosis. Endocr. J. 54, 649–657 (2007).
Huber, T.B. et al. Expression of functional CCR and CXCR chemokine receptors in podocytes. J. Immunol. 168, 6244–6252 (2002).
Burt, D. et al. The monocyte chemoattractant protein-1/cognate CC chemokine receptor 2 system affects cell motility in cultured human podocytes. Am. J. Pathol. 171, 1789–1799 (2007).
Fukuda, M. Versatile role of Rab27 in membrane trafficking: focus on the Rab27 effector families. J. Biochem. 137, 9–16 (2005).
Marcus, P.I. Dynamics of surface modification in myxovirus-infected cells. Cold Spring Harb. Symp. Quant. Biol. 27, 351–365 (1962).
Abercrombie, M., Heaysman, J.E. & Pegrum, S.M. The locomotion of fibroblasts in culture. II. RRuffling. Exp. Cell Res. 60, 437–444 (1970).
Abercrombie, M., Heaysman, J.E. & Pegrum, S.M. The locomotion of fibroblasts in culture. I. Movements of the leading edge. Exp. Cell Res. 59, 393–398 (1970).
Abercrombie, M., Heaysman, J.E. & Pegrum, S.M. The locomotion of fibroblasts in culture. 3. Movements of particles on the dorsal surface of the leading lamella. Exp. Cell Res. 62, 389–398 (1970).
Bretscher, M.S. & Thomson, J.N. Distribution of ferritin receptors and coated pits on giant HeLa cells. EMBO J. 2, 599–603 (1983).
Lee, J., Gustafsson, M., Magnusson, K.E. & Jacobson, K. The direction of membrane lipid flow in locomoting polymorphonuclear leukocytes. Science 247, 1229–1233 (1990).
Hopkins, C.R., Gibson, A., Shipman, M., Strickland, D.K. & Trowbridge, I.S. In migrating fibroblasts, recycling receptors are concentrated in narrow tubules in the pericentriolar area, and then routed to the plasma membrane of the leading lamella. J. Cell Biol. 125, 1265–1274 (1994).
Masztalerz, A. et al. Synaptotagmin 3 deficiency in T cells impairs recycling of the chemokine receptor CXCR4 and thereby inhibits CXCL12 chemokine-induced migration. J. Cell Sci. 120, 219–228 (2007).
Lawson, M.A. & Maxfield, F.R. Ca2+- and calcineurin-dependent recycling of an integrin to the front of migrating neutrophils. Nature 377, 75–79 (1995).
Li, Z. et al. Roles of PLC-β2 and -β3 and PI3Kγ in chemoattractant-mediated signal transduction. Science 287, 1046–1049 (2000).
Bach, T.L. et al. Phospholipase cβ is critical for T cell chemotaxis. J. Immunol. 179, 2223–2227 (2007).
Wei, C. et al. Calcium flickers steer cell migration. Nature 457, 901–905 (2009).
Proux-Gillardeaux, V., Raposo, G., Irinopoulou, T. & Galli, T. Expression of the Longin domain of TI-VAMP impairs lysosomal secretion and epithelial cell migration. Biol. Cell 99, 261–271 (2007).
Yoshinaga-Ohara, N., Takahashi, A., Uchiyama, T. & Sasada, M. Spatiotemporal regulation of moesin phosphorylation and rear release by Rho and serine/threonine phosphatase during neutrophil migration. Exp. Cell Res. 278, 112–122 (2002).
Lokuta, M.A. et al. Type Iγ PIP kinase is a novel uropod component that regulates rear retraction during neutrophil chemotaxis. Mol. Biol. Cell 18, 5069–5080 (2007).
Morin, N.A. et al. Nonmuscle myosin heavy chain IIA mediates integrin LFA-1 de-adhesion during T lymphocyte migration. J. Exp. Med. 205, 195–205 (2008).
Introne, W., Boissy, R.E. & Gahl, W.A. Clinical, molecular, and cell biological aspects of Chediak-Higashi syndrome. Mol. Genet. Metab. 68, 283–303 (1999).
Mundel, P. et al. Rearrangements of the cytoskeleton and cell contacts induce process formation during differentiation of conditionally immortalized mouse podocyte cell lines. Exp. Cell Res. 236, 248–258 (1997).
Kim, N.D., Chou, R.C., Seung, E., Tager, A.M. & Luster, A.D. A unique requirement for the leukotriene B4 receptor BLT1 for neutrophil recruitment in inflammatory arthritis. J. Exp. Med. 203, 829–835 (2006).
Moffat, J. et al. A lentiviral RNAi library for human and mouse genes applied to an arrayed viral high-content screen. Cell 124, 1283–1298 (2006).
Naldini, L. et al. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science 272, 263–267 (1996).
Wang, X. & Seed, B.A. PCR primer bank for quantitative gene expression analysis. Nucleic Acids Res. 31, e154 (2003).
Acknowledgements
We thank the Immune Circuits and RNAi Platform groups of the Broad Institute and W. Xu for discussions and the Imaging Core of the Ragon Institute of MGH, MIT and Harvard. Supported by the US National Institutes of Health (R01 DK074449 to A.D.L. and R01 GM064625 to N.W.A.), the Defense Advanced Research Projects Agency (W81XWH-04-C-0139 to N.H.), Fundação Luso-Americana para o Desenvolvimento (L.F.M.) and Fundação para a Ciência e a Tecnologia (L.F.M.).
Author information
Authors and Affiliations
Contributions
R.A.C. designed the experiments, did the screening, collected and analyzed data and wrote the manuscript; T.K.M. did air-pouch experiments, macrophage stimulation and GTPase assays and helped edit the manuscript; T.J.D. did confocal microscopy and analyzed lymphocytes; L.F.M. and C.M. helped with initial screens; R.P.F. assisted with air-pouch experiments; S.S. did confocal microscopy of podocytes; G.S.V.C. helped with experiments; T.A. and L.A.M. collected data; N.W.A. provided mouse strains; D.W. assisted with video migration assays of neutrophils; N.H. helped design experiments, analyzed data and helped edit the manuscript; A.D.L. helped design experiments, analyzed data and edited the manuscript; and all authors discussed results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–4, Supplementary Tables 1–2 and Supplementary Methods (PDF 836 kb)
Supplementary Video 1
Bone marrow derived neutrophils from wild-type C57BL/6 mice migrating in response to fMLP. (MOV 274 kb)
Supplementary Video 2
Bone marrow derived neutrophils from Syt7−/− mice migrating in response to fMLP. (MOV 148 kb)
Supplementary Video 3
CD4+ lymphocytes obtained from wild-type C57BL/6 mice, loaded with lysotracker red, migrating in response to CXCL10. (MOV 3861 kb)
Supplementary Video 4
CD4+ lymphocytes obtained from wild-type C57BL/6 mice, loaded with lysotracker red, migrating in response to CXCL10. (MOV 1135 kb)
Supplementary Video 5
CD4+ lymphocytes obtained from Syt7−/− mice, loaded with lysotracker red, migrating in response to CXCL10. (MOV 6688 kb)
Supplementary Video 6
CD4+ lymphocytes obtained from Syt7−/− mice, loaded with lysotracker red, migrating in response to CXCL10. (MOV 626 kb)
Rights and permissions
About this article
Cite this article
Colvin, R., Means, T., Diefenbach, T. et al. Synaptotagmin-mediated vesicle fusion regulates cell migration. Nat Immunol 11, 495–502 (2010). https://doi.org/10.1038/ni.1878
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.1878
This article is cited by
-
Protrudin-mediated ER-endosome contact sites promote phagocytosis
Cellular and Molecular Life Sciences (2023)
-
Sunitinib treatment promotes metastasis of drug-resistant renal cell carcinoma via TFE3 signaling pathway
Cell Death & Disease (2021)
-
The lysosomal Ragulator complex plays an essential role in leukocyte trafficking by activating myosin II
Nature Communications (2021)
-
Common transcriptome, plasma molecules, and imaging signatures in the aging brain and a Mendelian neurovascular disease, cerebral cavernous malformation
GeroScience (2020)
-
Eosinophil recruitment is dynamically regulated by interplay among lung dendritic cell subsets after allergen challenge
Nature Communications (2018)