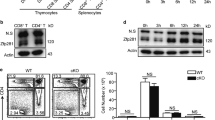
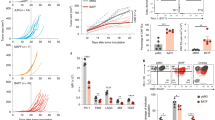
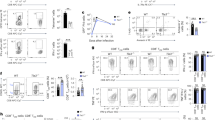
Abstract
The differentiation of activated CD4+ T cells into the T helper type 1 (TH1) or TH2 fate is regulated by cytokines and the transcription factors T-bet and GATA-3. Whereas interleukin 12 (IL-12) produced by antigen-presenting cells initiates the TH1 fate, signals that initiate the TH2 fate are not completely characterized. Here we show that early GATA-3 expression, required for TH2 differentiation, was induced by T cell factor 1 (TCF-1) and its cofactor β-catenin, mainly from the proximal Gata3 promoter upstream of exon 1b. This activity was induced after T cell antigen receptor (TCR) stimulation and was independent of IL-4 receptor signaling through the transcription factor STAT6. Furthermore, TCF-1 blocked TH1 fate by negatively regulating interferon-γ (IFN-γ) expression independently of β-catenin. Thus, TCF-1 initiates TH2 differentiation of activated CD4+ T cells by promoting GATA-3 expression and suppressing IFN-γ expression.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Glimcher, L.H. & Murphy, K.M. Lineage commitment in the immune system: the T helper lymphocyte grows up. Genes Dev. 14, 1693–1711 (2000).
Murphy, K.M. & Reiner, S.L. The lineage decisions of helper T cells. Nat. Rev. Immunol. 2, 933–944 (2002).
Corthay, A. A three-cell model for activation of naive T helper cells. Scand. J. Immunol. 64, 93–96 (2006).
Brown, D.R. et al. β2-microglobulin-dependent NK1.1+ T cells are not essential for T helper cell 2 immune responses. J. Exp. Med. 184, 1295–1304 (1996).
Ansel, K.M., Djuretic, I., Tanasa, B. & Rao, A. Regulation of Th2 differentiation and Il4 locus accessibility. Annu. Rev. Immunol. 24, 607–656 (2006).
Smiley, S.T., Kaplan, M.H. & Grusby, M.J. Immunoglobulin E production in the absence of interleukin-4-secreting CD1-dependent cells. Science 275, 977–979 (1997).
von der Weid, T., Beebe, A.M., Roopenian, D.C. & Coffman, R.L. Early production of IL-4 and induction of Th2 responses in the lymph node originate from an MHC class I-independent CD4+NK1.1− T cell population. J. Immunol. 157, 4421–4427 (1996).
Schmitz, J. et al. Induction of interleukin 4 (IL-4) expression in T helper (Th) cells is not dependent on IL-4 from non-Th cells. J. Exp. Med. 179, 1349–1353 (1994).
Ho, I.C., Hodge, M.R., Rooney, J.W. & Glimcher, L.H. The proto-oncogene c-maf is responsible for tissue-specific expression of interleukin-4. Cell 85, 973–983 (1996).
Zhang, D.H., Cohn, L., Ray, P., Bottomly, K. & Ray, A. Transcription factor GATA-3 is differentially expressed in murine Th1 and Th2 cells and controls Th2-specific expression of the interleukin-5 gene. J. Biol. Chem. 272, 21597–21603 (1997).
Zheng, W. & Flavell, R.A. The transcription factor GATA-3 is necessary and sufficient for Th2 cytokine gene expression in CD4 T cells. Cell 89, 587–596 (1997).
Zhu, J. et al. Conditional deletion of Gata3 shows its essential function in T(H)1-T(H)2 responses. Nat. Immunol. 5, 1157–1165 (2004).
Asnagli, H., Afkarian, M. & Murphy, K.M. Cutting edge: Identification of an alternative GATA-3 promoter directing tissue-specific gene expression in mouse and human. J. Immunol. 168, 4268–4271 (2002).
Scheinman, E.J. & Avni, O. Transcriptional regulation of GATA3 in T helper cells by the integrated activities of transcription factors downstream of the interleukin-4 receptor and T cell receptor. J. Biol. Chem. 284, 3037–3048 (2009).
Amsen, D. et al. Direct regulation of Gata3 expression determines the T helper differentiation potential of Notch. Immunity 27, 89–99 (2007).
Amsen, D. et al. Instruction of distinct CD4 T helper cell fates by different notch ligands on antigen-presenting cells. Cell 117, 515–526 (2004).
Fang, T.C. et al. Notch directly regulates Gata3 expression during T helper 2 cell differentiation. Immunity 27, 100–110 (2007).
Minter, L.M. et al. Inhibitors of γ-secretase block in vivo and in vitro T helper type 1 polarization by preventing Notch upregulation of Tbx21. Nat. Immunol. 6, 680–688 (2005).
Tanigaki, K. et al. Regulation of αβ/γδ T cell lineage commitment and peripheral T cell responses by Notch/RBP-J signaling. Immunity 20, 611–622 (2004).
Ong, C.T., Sedy, J.R., Murphy, K.M. & Kopan, R. Notch and presenilin regulate cellular expansion and cytokine secretion but cannot instruct Th1/Th2 fate acquisition. PLoS ONE 3, e2823 (2008).
Brantjes, H., Barker, N., van Es, J. & Clevers, H. TCF: Lady Justice casting the final verdict on the outcome of Wnt signalling. Biol. Chem. 383, 255–261 (2002).
Molenaar, M. et al. XTcf-3 transcription factor mediates β-catenin-induced axis formation in Xenopus embryos. Cell 86, 391–399 (1996).
Roose, J. et al. The Xenopus Wnt effector XTcf-3 interacts with Groucho-related transcriptional repressors. Nature 395, 608–612 (1998).
Staal, F.J., Burgering, B.M., van de Wetering, M. & Clevers, H.C. Tcf-1-mediated transcription in T lymphocytes: differential role for glycogen synthase kinase-3 in fibroblasts and T cells. Int. Immunol. 11, 317–323 (1999).
Staal, F.J., Luis, T.C. & Tiemessen, M.M. WNT signalling in the immune system: WNT is spreading its wings. Nat. Rev. Immunol. 8, 581–593 (2008).
Staal, F.J. & Sen, J.M. The canonical Wnt signaling pathway plays an important role in lymphopoiesis and hematopoiesis. Eur. J. Immunol. 38, 1788–1794 (2008).
Verbeek, S. et al. An HMG-box-containing T-cell factor required for thymocyte differentiation. Nature 374, 70–74 (1995).
Willinger, T. et al. Human naive CD8 T cells down-regulate expression of the WNT pathway transcription factors lymphoid enhancer binding factor 1 and transcription factor 7 (T cell factor-1) following antigen encounter in vitro and in vivo. J. Immunol. 176, 1439–1446 (2006).
Xu, Y., Banerjee, D., Huelsken, J., Birchmeier, W. & Sen, J.M. Deletion of β-catenin impairs T cell development. Nat. Immunol. 4, 1177–1182 (2003).
Staal, F.J. & Clevers, H.C. WNT signalling and haematopoiesis: a WNT-WNT situation. Nat. Rev. Immunol. 5, 21–30 (2005).
Yu, Q. & Sen, J.M. β-catenin regulates positive selection of thymocytes but not lineage commitment. J. Immunol. 178, 5028–5034 (2007).
Wu, B., Crampton, S.P. & Hughes, C.C. Wnt signaling induces matrix metalloproteinase expression and regulates T cell transmigration. Immunity 26, 227–239 (2007).
Moriyama, A. et al. GFP transgenic mice reveal active canonical Wnt signal in neonatal brain and in adult liver and spleen. Genesis 45, 90–100 (2007).
Kaplan, M.H., Schindler, U., Smiley, S.T. & Grusby, M.J. Stat6 is required for mediating responses to IL-4 and for development of Th2 cells. Immunity 4, 313–319 (1996).
Satoh, K. et al. Anteriorization of neural fate by inhibitor of β-catenin and T cell factor (ICAT), a negative regulator of Wnt signaling. Proc. Natl. Acad. Sci. USA 101, 8017–8021 (2004).
Hossain, M.Z., Yu, Q., Xu, M. & Sen, J.M. ICAT expression disrupts β-catenin-TCF interactions and impairs survival of thymocytes and activated mature T cells. Int. Immunol. 20, 925–935 (2008).
Mulroy, T., Xu, Y. & Sen, J.M. β-Catenin expression enhances generation of mature thymocytes. Int. Immunol. 15, 1485–1494 (2003).
Sekiya, T. et al. Identification of BMP and activin membrane-bound inhibitor (BAMBI), an inhibitor of transforming growth factor-β signaling, as a target of the β-catenin pathway in colorectal tumor cells. J. Biol. Chem. 279, 6840–6846 (2004).
Atcha, F.A. et al. A unique DNA binding domain converts T-cell factors into strong Wnt effectors. Mol. Cell. Biol. 27, 8352–8363 (2007).
Xu, M., Sharma, A., Wiest, D.L. & Sen, J.M. Pre-TCR-induced β-catenin facilitates traversal through beta-selection. J. Immunol. 182, 751–758 (2009).
Ding, Y., Shen, S., Lino, A.C., Curotto de Lafaille, M.A. & Lafaille, J.J. Beta-catenin stabilization extends regulatory T cell survival and induces anergy in nonregulatory T cells. Nat. Med. 14, 162–169 (2008).
Kalinski, P., Hilkens, C.M., Wierenga, E.A. & Kapsenberg, M.L. T-cell priming by type-1 and type-2 polarized dendritic cells: the concept of a third signal. Immunol. Today 20, 561–567 (1999).
Kalinski, P., Vieira, P.L., Schuitemaker, J.H., de Jong, E.C. & Kapsenberg, M.L. Prostaglandin E2 is a selective inducer of interleukin-12 p40 (IL-12p40) production and an inhibitor of bioactive IL-12p70 heterodimer. Blood 97, 3466–3469 (2001).
Woolard, M.D. et al. Francisella tularensis-infected macrophages release prostaglandin E2 that blocks T cell proliferation and promotes a Th2-like response. J. Immunol. 178, 2065–2074 (2007).
Shao, J., Jung, C., Liu, C. & Sheng, H. Prostaglandin E2 stimulates the β-catenin/T cell factor-dependent transcription in colon cancer. J. Biol. Chem. 280, 26565–26572 (2005).
Castellone, M.D., Teramoto, H., Williams, B.O., Druey, K.M. & Gutkind, J.S. Prostaglandin E2 promotes colon cancer cell growth through a Gs-axin-β-catenin signaling axis. Science 310, 1504–1510 (2005).
Maretto, S. et al. Mapping Wnt/β-catenin signaling during mouse development and in colorectal tumors. Proc. Natl. Acad. Sci. USA 100, 3299–3304 (2003).
Zheng, T. et al. Inducible targeting of IL-13 to the adult lung causes matrix metalloproteinase- and cathepsin-dependent emphysema. J. Clin. Invest. 106, 1081–1093 (2000).
Zhu, Z. et al. Pulmonary expression of interleukin-13 causes inflammation, mucus hypersecretion, subepithelial fibrosis, physiologic abnormalities, and eotaxin production. J. Clin. Invest. 103, 779–788 (1999).
Acknowledgements
We thank R. Wersto and team for cell sorting; the animal facility of the National Institute on Aging for maintaining animals; S. Luo and team for genotyping; H. Clevers (Hubrecht Institute) for TCF-1-deficient mice; W. Pear (University of Pennsylvania) for the dnMAML retroviral construct; and N. Taylor, A. Singer, R. Bosselut, R. Sen and A. Bhandoola for critically reading the manuscript. Supported by the Intramural Research Program of the National Institute on Aging of the National Institutes of Health and the Oak Ridge Institute for Science and Education's Research Associates Program of the National Institutes of Health.
Author information
Authors and Affiliations
Contributions
Q.Y. and J.M.S. designed and orchestrated the study and wrote the manuscript; Q.Y., A.S., M.Z.H., T.M.S. and K.E.L. did all the in vivo and in vitro experiments; S.Y.O., H.-G.M. and Z.Z. contributed to the analysis of OVA-injected mice in vivo; H.D. did electrophoretic mobility-shift assays; and B.W. and M.L.W. provided reagents and advice.
Corresponding author
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–8 and Supplementary Table 1 (PDF 1158 kb)
Rights and permissions
About this article
Cite this article
Yu, Q., Sharma, A., Oh, S. et al. T cell factor 1 initiates the T helper type 2 fate by inducing the transcription factor GATA-3 and repressing interferon-γ. Nat Immunol 10, 992–999 (2009). https://doi.org/10.1038/ni.1762
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni.1762
This article is cited by
-
TCF1 in T cell immunity: a broadened frontier
Nature Reviews Immunology (2022)
-
TCF-1: a maverick in T cell development and function
Nature Immunology (2022)
-
Transcriptional regulatory network for the establishment of CD8+ T cell exhaustion
Experimental & Molecular Medicine (2021)
-
Transcription tipping points for T follicular helper cell and T-helper 1 cell fate commitment
Cellular & Molecular Immunology (2021)
-
Induced regulatory T cells suppress Tc1 cells through TGF-β signaling to ameliorate STZ-induced type 1 diabetes mellitus
Cellular & Molecular Immunology (2021)