Abstract
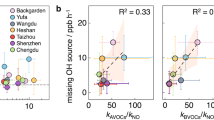
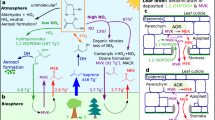
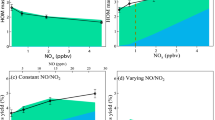
Most pollutants in the Earth’s atmosphere are removed by oxidation with highly reactive hydroxyl radicals. Field measurements have revealed much higher concentrations of hydroxyl radicals than expected in regions with high loads of the biogenic volatile organic compound isoprene1,2,3,4,5,6,7,8. Different isoprene degradation mechanisms have been proposed to explain the high levels of hydroxyl radicals observed5,9,10,11. Whether one or more of these mechanisms actually operates in the natural environment, and the potential impact on climate and air quality, has remained uncertain12,13,14. Here, we present a complete set of measurements of hydroxyl and peroxy radicals collected during isoprene-oxidation experiments carried out in an atmospheric simulation chamber, under controlled atmospheric conditions. We detected significantly higher concentrations of hydroxyl radicals than expected based on model calculations, providing direct evidence for a strong hydroxyl radical enhancement due to the additional recycling of radicals in the presence of isoprene. Specifically, our findings are consistent with the unimolecular reactions of isoprene-derived peroxy radicals postulated by quantum chemical calculations9,10,11. Our experiments suggest that more than half of the hydroxyl radicals consumed in isoprene-rich regions, such as forests, are recycled by these unimolecular reactions with isoprene. Although such recycling is not sufficient to explain the high concentrations of hydroxyl radicals observed in the field, we conclude that it contributes significantly to the oxidizing capacity of the atmosphere in isoprene-rich regions.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Carslaw, N. et al. OH and HO2 radical chemistry in a forested region of north-western Greece. Atmos. Environ. 35, 4725–4737 (2001).
Tan, D. et al. HOX budget in a deciduous forest: results from the PROPHET summer 1998 campaign. J. Geophys. Res. 106, 24407–24427 (2001).
Kuhn, U. et al. Isoprene and monoterpene fluxes from Central Amazonian rainforest inferred from tower-based and airborne measurements, and implications on the atmospheric chemistry and the local carbon budget. Atmos. Chem. Phys. 7, 2855–2879 (2007).
Ren, X. et al. HOX chemistry during INTEX-A 2004: Observation, model calculation, and comparison with previous studies. J. Geophys. Res. 113, D05310 (2008).
Lelieveld, J. et al. Atmospheric oxidation capacity sustained by a tropical forest. Nature 452, 737–740 (2008).
Hofzumahaus, A. et al. Amplified trace gas removal in the troposphere. Science 324, 1702–1704 (2009).
Whalley, L. K. et al. Quantifying the magnitude of a missing hydroxyl radical source in a tropical rainforest. Atmos. Chem. Phys. 11, 7223–7233 (2011).
Wolfe, G. M. et al. The chemistry of atmosphere-forest exchange (CAFE) model—part 2: Application to BEARPEX-2007 observations. Atmos. Chem. Phys. 11, 1269–1294 (2011).
Peeters, J., Nguyen, T. L. & Vereecken, L. HOX radical regeneration in the oxidation of isoprene. Phys. Chem. Chem. Phys. 11, 5935–5939 (2009).
Peeters, J. & Müller, J-F. HOX radical regeneration in isoprene oxidation via peroxy radical isomerisations. II: Experimental evidence and global impact. Phys. Chem. Chem. Phys. 12, 14227–14235 (2010).
Da Silva, G., Graham, C. & Wang, Z-F. Unimolecular β-hydroxyperoxy radical decomposition with OH recycling in the photochemical oxidation of isoprene. Environ. Sci. Technol. 44, 250–256 (2010).
Stavrakou, T., Peeters, J. & Müller, J. F. Improved global modelling of HOX recycling in isoprene oxidation: Evaluation against the GABRIEL and INTEX-A aircraft campaign measurements. Atmos. Chem. Phys. 10, 9863–9878 (2010).
Archibald, A. T. et al. Impacts of HOX regeneration and recycling in the oxidation of isoprene: Consequences for the composition of past, present and future atmospheres. Geophys. Res. Lett. 38, L05804 (2011).
Taraborrelli, D. et al. Hydroxyl radical buffered by isoprene oxidation over tropical forests. Nature Geosci. 5, 190–193 (2012).
Guenther, A. et al. A global model of natural volatile organic compound emissions. J. Geophys. Res. 100, 8873–8892 (1995).
Goldstein, A. H. & Galbally, I. E. Known and unexplored organic constituents in the earth’s atmosphere. Environ. Sci. Technol. 41, 1514–1521 (2007).
Paulot, F. et al. Unexpected epoxide formation in the gas-phase photooxidation of isoprene. Science 325, 730–733 (2009).
Lu, K. D. et al. Observation and modelling of OH and HO2 concentrations in the Pearl River Delta 2006: A missing OH source in a VOC rich atmosphere. Atmos. Chem. Phys. 12, 1541–1569 (2012).
Lu, K. D. et al. Missing OH source in a suburban environment near Beijing: Observed and modelled OH and HO2 concentrations in summer 2006. Atmos. Chem. Phys. 13, 1057–1080 (2013).
Stone, D. et al. Isoprene oxidation mechanisms: Measurements and modelling of OH and HO2 over a South-East Asian tropical rainforest during the OP3 field campaign. Atmos. Chem. Phys. 11, 6749–6771 (2011).
Thornton, J. A. et al. Ozone production rates as a function of NOX abundances and HOX production rates in the Nashville urban plume. J. Geophys. Res. 107, ACH 7-1–ACH 7-17 (2002).
Jenkin, M. E., Saunders, S. M. & Pilling, M. J. The tropospheric degradation of volatile organic compounds: A protocol for mechanism development. Atmos. Environ. 31, 81–104 (1997).
Saunders, S. M., Jenkin, M. E., Derwent, R. G. & Pilling, M. J. Protocol for the development of the Master Chemical Mechanism, MCMv3 (Part A): Tropospheric degradation of non-aromatic volatile organic compounds. Atmos. Chem. Phys. 3, 161–180 (2003).
Fuchs, H. et al. Comparison of OH concentration measurements by DOAS and LIF during SAPHIR chamber experiments at high OH reactivity and low NO concentration. Atmos. Meas. Tech. 5, 1611–1626 (2012).
Wolfe, G. M. et al. Photolysis, OH reactivity and ozone reactivity of a proxy for isoprene-derived hydroperoxyenals. Phys. Chem. Chem. Phys. 14, 7276–7286 (2012).
Crounse, J. D., Paulot, F., Kjaergaard, H. G. & Wennberg, P. O. Peroxy radical isomerization in the oxidation of isoprene. Phys. Chem. Chem. Phys. 13, 13607–13613 (2011).
Zhang, H. & Kamens, R. The influence of isoprene peroxy radical isomerization mechanisms on ozone simulation with the presence of NOX . J. Atmos. Chem. 69, 67–81 (2012).
Archibald, A. T. et al. Impacts of mechanistic changes on HOX formation and recycling in the oxidation of isoprene. Atmos. Chem. Phys. 10, 8097–8118 (2010).
Kubistin, D. et al. Hydroxyl radicals in the tropical troposphere over the Suriname rainforest: Comparison of measurements with the box model MECCA. Atmos. Chem. Phys. 10, 9705–9728 (2010).
Crounse, J. D. et al. On the atmospheric fate of methacrolein: 1. Peroxy radical isomerization following addition of OH and O2 . J. Phys. Chem. A 116, 5756–5762 (2012).
Acknowledgements
This work was supported by the EU FP-7 program EUROCHAMP-2 (grant agreement No. 228335) and by the EU FP-7 program PEGASOS (grant agreement No. 265307). S. Nehr and B. Bohn thank the Deutsche Forschungsgemeinschaft for financial support (grant agreement No. BO 1580/3-1). K. D. Lu thanks the National Natural Science Foundation of China (Major Program: 21190052) for financial support. The authors thank A. Buchholz, P. Schlag, F. Rubach, H-C. Wu, S. Dixneuf, M. Vietz, P. Müsgen and M. Bachner for additional measurements during this campaign and technical support.
Author information
Authors and Affiliations
Contributions
F.R., H.F. and B.B. designed experiments. H.F. performed model calculations for SAPHIR experiments. K.L. carried out model runs with updated isoprene chemistry for PRIDE-PRD2006. H.F. and A.H. wrote the manuscript. All authors contributed to the study with measurements, discussed results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 2818 kb)
Rights and permissions
About this article
Cite this article
Fuchs, H., Hofzumahaus, A., Rohrer, F. et al. Experimental evidence for efficient hydroxyl radical regeneration in isoprene oxidation. Nature Geosci 6, 1023–1026 (2013). https://doi.org/10.1038/ngeo1964
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ngeo1964
This article is cited by
-
Mechanism of secondary organic aerosol formation from the reaction of isoprene with sulfoxy radicals
Environmental Science and Pollution Research (2021)
-
Satellite isoprene retrievals constrain emissions and atmospheric oxidation
Nature (2020)
-
Impact on short-lived climate forcers increases projected warming due to deforestation
Nature Communications (2018)
-
Maximum efficiency in the hydroxyl-radical-based self-cleansing of the troposphere
Nature Geoscience (2014)
-
Radical regeneration from isoprene
Nature Geoscience (2013)