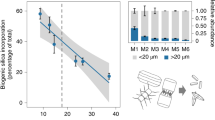
Abstract
The marine silicon cycle is thought to be intimately tied to the carbon cycle through its effect on the growth of diatoms. These unicellular algae form substantial blooms in cold, nutrient-rich waters. Their dense, siliceous cell walls promote the sinking of particulate matter, and all the carbon and nutrients contained therein1. As such, diatoms are thought to be the primary organisms responsible for the low levels of dissolved silicon observed in the surface ocean and the export of mineral silica to depth. Here, we use synchrotron X-ray fluorescence microscopy to determine the elemental composition of individual diatoms and cyanobacterial cells from the eastern equatorial Pacific and the Sargasso Sea. We show that cells of Synechococcus, a small unicellular marine cyanobacterium that dominates in nutrient-depleted waters2, can exhibit cellular ratios of silicon to sulphur, and silicon to phosphorus, approaching those detected in diatoms in the same location. Silicon accumulation was also observed in cultured Synechococcus strains. We estimate that the water column inventory of silicon in Synechococcus can exceed that of diatoms in some cases. We suggest that picocyanobacteria may exert a previously unrecognized influence on the oceanic silicon cycle, especially in nutrient-poor waters.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Buesseler, K. O. The decoupling of production and particulate export in the surface ocean. Glob. Biogeochem. Cycles 12, 297–310 (1998).
Agawin, N. S. R., Duarte, C. M. & Agusti, S. Nutrient and temperature control of the contribution of picoplankton to phytoplankton biomass and production. Limnol. Oceanogr. 45, 591–600 (2000).
Twining, B. S., Nunez-Milland, D., Vogt, S., Johnson, R. S. & Sedwick, P. N. Variations in Synechococcus cell quotas of phosphorus, sulfur, manganese, iron, nickel, and zinc within mesoscale eddies in the Sargasso Sea. Limnol. Oceanogr. 55, 492–506 (2010).
Krivtsov, V., Bellinger, E. G. & Sigee, D. C. Elemental composition of Microcystis aeruginosa under conditions of lake nutrient depletion. Aquat. Ecol. 39, 123–134 (2005).
Sigee, D. C. & Levado, E. Cell surface elemental composition of Microcystis aeruginosa: High-Si and low-Si subpopulations within the water column of a eutrophic lake. J. Plankt. Res. 22, 2137–2153 (2000).
Hirota, R., Hata, Y., Ikeda, T., Ishida, T. & Kuroda, A. The silicon layer supports acid resistance of Bacillus cereus spores. J. Bacteriol. 192, 111–116 (2010).
Baines, S. B., Twining, B. S., Brzezinski, M. A., Nelson, D. M. & Fisher, N. S. Regional differences in silicification of marine diatoms and their biogeochemical implications. Glob. Biogeochem. Cycles 24, GB4031 (2010).
Hasle, G. R. & Syvertsen, E. E. in Identifying Marine Diatoms and Dinoflagellates (ed. Tomas, C. R.) 5–385 (Academic, 1997).
Price, N. M. et al. Preparation and chemistry of the artificial algal media medium Aquil. Biol. Oceanogr. 6, 443–461 (1988–1989).
Rickert, D., Schluter, M. & Wallmann, K. Dissolution kinetics of biogenic silica from the water column to the sediments. Geochim. et Cosmochim. Acta 66, 439–455 (2002).
Schultze-Lam, S., Harauz, G. & Beveridge, T. J. Participation of a cyanobacterial S-layer in fine-grain mineral formation. J. Bacteriol. 174, 7971–7981 (1992).
Nelson, D. M., Riedel, G. F., Millan-Nunez, R. & Lara-Lara, J. R. Silicon uptake by algae with no known Si requirement. I. True cellular uptake and pH induced precipitation by Phaeodactylum tricornutum (Bacillariophyceae) and Platymonas sp. (Prasinophyceae). J. Phycol. 20, 141–147 (1984).
Benning, L. G., Phoenix, V. R., Yee, N. & Tobin, M. J. Molecular characterization of cyanobacterial silicification using synchrotron infrared micro-spectroscopy. Geochim. Cosmochim. Acc. 68, 729–741 (2004).
Bidle, K. D. & Azam, F. Accelerated dissolution of diatom silica by marine bacterial assemblages. Nature 397, 508–512 (1999).
Yee, N., Phoenix, V. R., Konhauser, K. O., Benning, L. G. & Ferris, F. G. The effect of cyanobacteria on silica precipitation at neutral pH: Implications for bacterial silicification in geothermal hot springs. Chem. Geol. 199, 83–90 (2003).
Martin-Jezequel, V., Hildebrand, M. & Brzezinski, M. A. Silicon metabolism in diatoms: Implications for growth. J. Phycol. 36, 821–840 (2000).
Krause, J. W. et al. The effects of biogenic silica detritus, zooplankton grazing, and diatom-size structure on Si-cycling in the euphotic zone of the eastern equatorial Pacific. Limnol. Oceanogr. 55, 2608–2622 (2010).
Ragueneau, O. & Treguer, P. Determination of biogenic silica in coastal waters—applicability and limits of the alkaline digestion method. Mar. Chem. 45, 43–51 (1994).
Sarmiento, J. L., Gruber, N., Brzezinski, M. A. & Dunne, J. P. High-latitude controls of thermocline nutrients and low latitude biological productivity. Nature 427, 56–60 (2004).
Lomas, M. L. & Moran, S. B. Evidence for aggregation and export of cyanobacteria and nano-eukaryotes from the Sargasso Sea euphotic zone. Biogeosciences 8, 203–216 (2011).
Richardson, T. L. & Jackson, G. A. Small phytoplankton and carbon export from the surface ocean. Science 315, 838–840 (2007).
Stukel, M. R. & Landry, M. R. Contribution of picophytoplankton to carbon export in the equatorial Pacific: A reassessment of food web flux inferences from inverse models. Limnol. Oceanogr. 55, 2669–2685 (2010).
Twining, B. S. et al. Metal quotas of plankton in the equatorial Pacific Ocean. Deep Sea Res. II 58, 325–341 (2011).
Vogt, S. MAPS: A set of software tools for analysis and visualization of 3D X-ray fluorescence data sets. J. Phys. IV 104, 635–638 (2003).
Núñez-Milland, D. R., Baines, S. B., Vogt, S. & Twining, B. S. Quantification of phosphorus in single cells using synchrotron X-ray fluorescence. J. Synchrotron Radiat. 17, 560–566 (2010).
Twining, B. S. et al. Quantifying trace elements in individual aquatic protist cells with a synchrotron X-ray fluorescence microprobe. Anal. Chem. 75, 3806–3816 (2003).
Taylor, A. G., Landry, M. R., Selph, K. E. & Yang, E. J. Biomass, size structure and depth distributions of the microbial community in the eastern equatorial Pacific. Deep Sea Res. II 58, 342–357 (2011).
Guillard, R. R. & Ryther, J. H. Studies of marine planktonic diatoms.1. Cyclotella nana hustedt, and Detonula confervacea (Cleve) Gran. Can. J. Microbiol. 8, 229–239 (1962).
Brzezinski, M. A. & Nelson, D. M. The annual silica cycle in the Sargasso Sea near Bermuda. Deep-Sea Res. I 42, 1215–1237 (1995).
Acknowledgements
This work was supported by the NSF (CBET 0730061 and OCE 0527062 to B.S.T., OCE 527059 and OCE 0966201 to S.B.B.). H.M. was supported by an NSF REU grant to Bigelow Laboratory (OCE 0755142). D.A. was supported by the Simons Summer Research Fellowship at Stony Brook University. Use of the Advanced Photon Source, an Office of Science User Facility operated for the US Department of Energy (DOE) Office of Science by Argonne National Laboratory, was supported by the US DOE under Contract No. DE-AC02-06CH11357.
Author information
Authors and Affiliations
Contributions
S.B.B. and B.S.T. were responsible for the initial idea for the paper, and both were most involved in the writing and figure preparation, with substantial input from M.A.B. and J.W.K. All SXRF analyses were conducted by B.S.T. and S.B.B. S.V. provided logistics at the 2-ID-E microprobe at the Advanced Photon Source and oversaw analysis for X-ray fluorescence spectra. S.B.B., B.S.T., J.W.K. and M.A.B. all oversaw the various culture experiments. D.A. and H.M. were both involved in designing, conducting and analysing the culture experiments. J.W.K. and M.A.B. provided analyses of biogenic and lithogenic silica for the Sargasso samples that were collected by B.S.T.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 382 kb)
Rights and permissions
About this article
Cite this article
Baines, S., Twining, B., Brzezinski, M. et al. Significant silicon accumulation by marine picocyanobacteria. Nature Geosci 5, 886–891 (2012). https://doi.org/10.1038/ngeo1641
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ngeo1641
This article is cited by
-
Holistic view of the seascape dynamics and environment impact on macro-scale genetic connectivity of marine plankton populations
BMC Ecology and Evolution (2023)
-
About Method for Testing Bioavailable Si in Aqueous Samples
Silicon (2023)
-
Evidence of the Significant Contribution of Heterotrophic Diazotrophs to Nitrogen Fixation in the Eastern Indian Ocean During Pre-Southwest Monsoon Period
Ecosystems (2022)
-
Significant Pico- and Nanoplankton Contributions to Biogenic Silica Standing Stocks and Production Rates in the Oligotrophic Eastern Indian Ocean
Ecosystems (2021)
-
Environmental factors controlling the dynamics of phytoplankton communities during spring and fall seasons in the southern Sunda Shelf
Environmental Science and Pollution Research (2020)