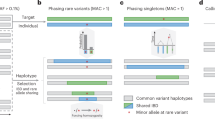
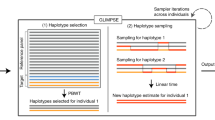
Abstract
Haplotype phasing is a fundamental problem in medical and population genetics. Phasing is generally performed via statistical phasing in a genotyped cohort, an approach that can yield high accuracy in very large cohorts but attains lower accuracy in smaller cohorts. Here we instead explore the paradigm of reference-based phasing. We introduce a new phasing algorithm, Eagle2, that attains high accuracy across a broad range of cohort sizes by efficiently leveraging information from large external reference panels (such as the Haplotype Reference Consortium; HRC) using a new data structure based on the positional Burrows-Wheeler transform. We demonstrate that Eagle2 attains a ∼20× speedup and ∼10% increase in accuracy compared to reference-based phasing using SHAPEIT2. On European-ancestry samples, Eagle2 with the HRC panel achieves >2× the accuracy of 1000 Genomes–based phasing. Eagle2 is open source and freely available for HRC-based phasing via the Sanger Imputation Service and the Michigan Imputation Server.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Tewhey, R., Bansal, V., Torkamani, A., Topol, E.J. & Schork, N.J. The importance of phase information for human genomics. Nat. Rev. Genet. 12, 215–223 (2011).
Browning, S.R. & Browning, B.L. Haplotype phasing: existing methods and new developments. Nat. Rev. Genet. 12, 703–714 (2011).
Stephens, M., Smith, N.J. & Donnelly, P. A new statistical method for haplotype reconstruction from population data. Am. J. Hum. Genet. 68, 978–989 (2001).
Halperin, E. & Eskin, E. Haplotype reconstruction from genotype data using Imperfect Phylogeny. Bioinformatics 20, 1842–1849 (2004).
Stephens, M. & Scheet, P. Accounting for decay of linkage disequilibrium in haplotype inference and missing-data imputation. Am. J. Hum. Genet. 76, 449–462 (2005).
Scheet, P. & Stephens, M. A fast and flexible statistical model for large-scale population genotype data: applications to inferring missing genotypes and haplotypic phase. Am. J. Hum. Genet. 78, 629–644 (2006).
Browning, S.R. & Browning, B.L. Rapid and accurate haplotype phasing and missing-data inference for whole-genome association studies by use of localized haplotype clustering. Am. J. Hum. Genet. 81, 1084–1097 (2007).
Kong, A. et al. Detection of sharing by descent, long-range phasing and haplotype imputation. Nat. Genet. 40, 1068–1075 (2008).
Browning, B.L. & Browning, S.R. A unified approach to genotype imputation and haplotype-phase inference for large data sets of trios and unrelated individuals. Am. J. Hum. Genet. 84, 210–223 (2009).
Delaneau, O., Marchini, J. & Zagury, J.-F. A linear complexity phasing method for thousands of genomes. Nat. Methods 9, 179–181 (2011).
Williams, A.L., Patterson, N., Glessner, J., Hakonarson, H. & Reich, D. Phasing of many thousands of genotyped samples. Am. J. Hum. Genet. 91, 238–251 (2012).
Delaneau, O., Zagury, J.-F. & Marchini, J. Improved whole-chromosome phasing for disease and population genetic studies. Nat. Methods 10, 5–6 (2013).
Loh, P.-R., Palamara, P.F. & Price, A.L. Fast and accurate long-range phasing in a UK Biobank cohort. Nat. Genet. 48, 811–816 (2016).
O'Connell, J. et al. Haplotype estimation for biobank-scale data sets. Nat. Genet. 48, 817–820 (2016).
Snyder, M.W., Adey, A., Kitzman, J.O. & Shendure, J. Haplotype-resolved genome sequencing: experimental methods and applications. Nat. Rev. Genet. 16, 344–358 (2015).
van de Geijn, B., McVicker, G., Gilad, Y. & Pritchard, J.K. WASP: allele-specific software for robust molecular quantitative trait locus discovery. Nat. Methods 12, 1061–1063 (2015).
Kumasaka, N., Knights, A.J. & Gaffney, D.J. Fine-mapping cellular QTLs with RASQUAL and ATAC–seq. Nat. Genet. 48, 206–213 (2016).
Das, S. et al. Next-generation genotype imputation service and methods. Nat. Genet. http://dx.doi.org/10.1038/ng.3656 (published online 29 August, 2016).
McCarthy, S. et al. A reference panel of 64,976 haplotypes for genotype imputation. Nat. Genet. http://dx.doi.org/10.1038/ng.3643 (published online 22 August 2016).
Durbin, R. Efficient haplotype matching and storage using the positional Burrows–Wheeler transform (PBWT). Bioinformatics 30, 1266–1272 (2014).
Li, N. & Stephens, M. Modeling linkage disequilibrium and identifying recombination hotspots using single-nucleotide polymorphism data. Genetics 165, 2213–2233 (2003).
Sudlow, C. et al. UK Biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 12, e1001779 (2015).
Kvale, M.N. et al. Genotyping informatics and quality control for 100,000 subjects in the Genetic Epidemiology Research on Adult Health and Aging (GERA) cohort. Genetics 200, 1051–1060 (2015).
Banda, Y. et al. Characterizing race/ethnicity and genetic ancestry for 100,000 subjects in the Genetic Epidemiology Research on Adult Health and Aging (GERA) cohort. Genetics 200, 1285–1295 (2015).
Browning, B.L. & Browning, S.R. Genotype imputation with millions of reference samples. Am. J. Hum. Genet. 98, 116–126 (2016).
1000 Genomes Project Consortium. A global reference for human genetic variation. Nature 526, 68–74 (2015).
Howie, B., Fuchsberger, C., Stephens, M., Marchini, J. & Abecasis, G.R. Fast and accurate genotype imputation in genome-wide association studies through pre-phasing. Nat. Genet. 44, 955–959 (2012).
He, D., Han, B. & Eskin, E. Hap-seq: an optimal algorithm for haplotype phasing with imputation using sequencing data. J. Comput. Biol. 20, 80–92 (2013).
Delaneau, O., Howie, B., Cox, A.J., Zagury, J.-F. & Marchini, J. Haplotype estimation using sequencing reads. Am. J. Hum. Genet. 93, 687–696 (2013).
Sharp, K., Kretzschmar, W., Delaneau, O. & Marchini, J. Phasing for medical sequencing using rare variants and large haplotype reference panels. Bioinformatics 32, 1974–1980 (2016).
Chang, C.C. et al. Second-generation PLINK: rising to the challenge of larger and richer datasets. Gigascience 4, 7 (2015).
Acknowledgements
We are grateful to S. Linderman, N. Patterson, L. O'Connor, A. Gusev, and B. van de Geijn for helpful discussions. This research was conducted using the UK Biobank Resource. P.L., P.P., and A.L.P. were supported by US National Institutes of Health grants R01 HG006399 and R01 MH101244 and fellowship F32 HG007805. P.D., S.M., R.D., and the Sanger Institute HRC server were supported by Wellcome Trust grant WT098051. C.F., G.R.A., and the Michigan Imputation Server were supported by the Austrian Science Fund (FWF) grant J-3401 and US National Institutes of Health grants HG007022 and HL117626. H.K.F. was supported by the Fannie and John Hertz Foundation. Computational analyses were performed on the Orchestra High Performance Compute Cluster at Harvard Medical School, which is partially supported by grant NCRR 1S10RR028832-01, and on the Lisa Genetic Cluster Computer hosted by SURFsara and financially supported by the Netherlands Scientific Organization (NWO 480-05-003, principal investigator D. Posthuma) along with a supplement from the Dutch Brain Foundation and the VU University Amsterdam.
Author information
Authors and Affiliations
Contributions
P.-R.L. and A.L.P. designed the study. P.-R.L., P.F.P., Y.A.R., and H.K.F. developed the algorithm. P.-R.L. wrote the software. P.-R.L. and P.D. performed experiments. P.D. and S.M. incorporated the software into the Sanger Imputation Service. C.F., S.S., and L.F. incorporated the software into the Michigan Imputation Server. All authors analyzed data and wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1 and 2, Supplementary Tables 1–13 and Supplementary Note. (PDF 1968 kb)
Rights and permissions
About this article
Cite this article
Loh, PR., Danecek, P., Palamara, P. et al. Reference-based phasing using the Haplotype Reference Consortium panel. Nat Genet 48, 1443–1448 (2016). https://doi.org/10.1038/ng.3679
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng.3679
This article is cited by
-
Exploratory study of cold hypersensitivity in Japanese women: genetic associations and somatic symptom burden
Scientific Reports (2024)
-
Transposable elements mediate genetic effects altering the expression of nearby genes in colorectal cancer
Nature Communications (2024)
-
Genetic evidence for T-wave area from 12-lead electrocardiograms to monitor cardiovascular diseases in patients taking diabetes medications
Human Genetics (2024)
-
Genome-wide association and Mendelian randomization analysis provide insights into the shared genetic architecture between high-dimensional electrocardiographic features and ischemic heart disease
Human Genetics (2024)
-
Selection, optimization and validation of ten chronic disease polygenic risk scores for clinical implementation in diverse US populations
Nature Medicine (2024)