Abstract
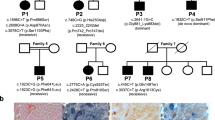
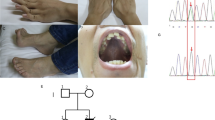
Known disease mechanisms in mitochondrial DNA (mtDNA) maintenance disorders alter either the mitochondrial replication machinery (POLG, POLG2 and C10orf2)1,2,3 or the biosynthesis pathways of deoxyribonucleoside 5′-triphosphates for mtDNA synthesis4,5,6,7,8,9,10,11. However, in many of these disorders, the underlying genetic defect has yet to be discovered. Here, we identify homozygous nonsense and missense mutations in the orphan gene C20orf72 in three families with a mitochondrial syndrome characterized by external ophthalmoplegia, emaciation and respiratory failure. Muscle biopsies showed mtDNA depletion and multiple mtDNA deletions. C20orf72, hereafter MGME1 (mitochondrial genome maintenance exonuclease 1), encodes a mitochondrial RecB-type exonuclease belonging to the PD–(D/E)XK nuclease superfamily. We show that MGME1 cleaves single-stranded DNA and processes DNA flap substrates. Fibroblasts from affected individuals do not repopulate after chemically induced mtDNA depletion. They also accumulate intermediates of stalled replication and show increased levels of 7S DNA, as do MGME1-depleted cells. Thus, we show that MGME1-mediated mtDNA processing is essential for mitochondrial genome maintenance.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Accession codes
Accessions
NCBI Reference Sequence
References
Van Goethem, G. et al. Mutation of POLG is associated with progressive external ophthalmoplegia characterized by mtDNA deletions. Nat. Genet. 28, 211–212 (2001).
Longley, M.J. et al. Mutant POLG2 disrupts DNA polymerase γ subunits and causes progressive external ophthalmoplegia. Am. J. Hum. Genet. 78, 1026–1034 (2006).
Spelbrink, J.N. et al. Human mitochondrial DNA deletions associated with mutations in the gene encoding Twinkle, a phage T7 gene 4–like protein localized in mitochondria. Nat. Genet. 28, 223–231 (2001).
Nishino, I., Spinazzola, A. & Hirano, M. Thymidine phosphorylase gene mutations in MNGIE, a human mitochondrial disorder. Science 283, 689–692 (1999).
Kaukonen, J. et al. Role of adenine nucleotide translocator 1 in mtDNA maintenance. Science 289, 782–785 (2000).
Mandel, H. et al. The deoxyguanosine kinase gene is mutated in individuals with depleted hepatocerebral mitochondrial DNA. Nat. Genet. 29, 337–341 (2001).
Saada, A. et al. Mutant mitochondrial thymidine kinase in mitochondrial DNA depletion myopathy. Nat. Genet. 29, 342–344 (2001).
Elpeleg, O. et al. Deficiency of the ADP-forming succinyl-CoA synthase activity is associated with encephalomyopathy and mitochondrial DNA depletion. Am. J. Hum. Genet. 76, 1081–1086 (2005).
Spinazzola, A. et al. MPV17 encodes an inner mitochondrial membrane protein and is mutated in infantile hepatic mitochondrial DNA depletion. Nat. Genet. 38, 570–575 (2006).
Bourdon, A. et al. Mutation of RRM2B, encoding p53-controlled ribonucleotide reductase (p53R2), causes severe mitochondrial DNA depletion. Nat. Genet. 39, 776–780 (2007).
Ostergaard, E. et al. Deficiency of the α subunit of succinate–coenzyme A ligase causes fatal infantile lactic acidosis with mitochondrial DNA depletion. Am. J. Hum. Genet. 81, 383–387 (2007).
Haack, T.B. et al. Molecular diagnosis in mitochondrial complex I deficiency using exome sequencing. J. Med. Genet. 49, 277–283 (2012).
Elstner, M. et al. MitoP2: an integrative tool for the analysis of the mitochondrial proteome. Mol. Biotechnol. 40, 306–315 (2008).
Calvo, S.E. et al. Molecular diagnosis of infantile mitochondrial disease with targeted next-generation sequencing. Sci. Transl. Med. 4, 118ra10 (2012).
Pagliarini, D.J. et al. A mitochondrial protein compendium elucidates complex I disease biology. Cell 134, 112–123 (2008).
Steczkiewicz, K. et al. Sequence, structure and functional diversity of PD-(D/E)XK phosphodiesterase superfamily. Nucleic Acids Res. 40, 7016–7045 (2012).
Aravind, L. et al. Holliday junction resolvases and related nucleases: identification of new families, phyletic distribution and evolutionary trajectories. Nucleic Acids Res. 28, 3417–3432 (2000).
Singleton, M.R. et al. Crystal structure of RecBCD enzyme reveals a machine for processing DNA breaks. Nature 432, 187–193 (2004).
Holt, I.J. et al. Coupled leading- and lagging-strand synthesis of mammalian mitochondrial DNA. Cell 100, 515–524 (2000).
Liu, P. et al. Removal of oxidative DNA damage via FEN1-dependent long-patch base excision repair in human cell mitochondria. Mol. Cell. Biol. 28, 4975–4987 (2008).
Brown, T.A. & Clayton, D.A. Release of replication termination controls mitochondrial DNA copy number after depletion with 2′,3′-dideoxycytidine. Nucleic Acids Res. 30, 2004–2010 (2002).
Stewart, J.D. et al. POLG mutations cause decreased mitochondrial DNA repopulation rates following induced depletion in human fibroblasts. Biochim. Biophys. Acta 1812, 321–325 (2011).
Wanrooij, S. et al. Expression of catalytic mutants of the mtDNA helicase Twinkle and polymerase POLG causes distinct replication stalling phenotypes. Nucleic Acids Res. 35, 3238–3251 (2007).
Copeland, W.C. & Longley, M.J. DNA2 resolves expanding flap in mitochondrial base excision repair. Mol. Cell 32, 457–458 (2008).
Zheng, L. et al. Human DNA2 is a mitochondrial nuclease/helicase for efficient processing of DNA replication and repair intermediates. Mol. Cell 32, 325–336 (2008).
Duxin, J.P. et al. Human Dna2 is a nuclear and mitochondrial DNA maintenance protein. Mol. Cell. Biol. 29, 4274–4282 (2009).
Tann, A.W. et al. Apoptosis induced by persistent single-strand breaks in mitochondrial genome: critical role of EXOG (5′-EXO/endonuclease) in their repair. J. Biol. Chem. 286, 31975–31983 (2011).
Pohjoismäki, J.L. et al. Mammalian mitochondrial DNA replication intermediates are essentially duplex but contain extensive tracts of RNA/DNA hybrid. J. Mol. Biol. 397, 1144–1155 (2010).
McKenna, A. et al. The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 20, 1297–1303 (2010).
Zsurka, G. et al. Clonally expanded mitochondrial DNA mutations in epileptic individuals with mutated DNA polymerase γ. J. Neuropathol. Exp. Neurol. 67, 857–866 (2008).
Danhauser, K. et al. Cellular rescue-assay aids verification of causative DNA-variants in mitochondrial complex I deficiency. Mol. Genet. Metab. 103, 161–166 (2011).
Rorbach, J. et al. PDE12 removes mitochondrial RNA poly(A) tails and controls translation in human mitochondria. Nucleic Acids Res. 39, 7750–7763 (2011).
Reyes, A. et al. Analysis of replicating mitochondrial DNA by two-dimensional agarose gel electrophoresis. Methods Mol. Biol. 372, 219–232 (2007).
Acknowledgements
T.J.N. and M. Minczuk are grateful to S. Wood and I. Holt for stimulating discussions during the course of this work. We are grateful to S. Beyer, K. Kappes-Horn, M. Stepien-Mering, E. Botz and R. Hellinger for technical assistance. We thank R. Wiesner (University of Cologne) for providing the TFAM antibody. This work was supported by the Medical Research Council UK (T.J.N., J.R. and M. Minczuk) and the German Bundesministerium für Bildung und Forschung (BMBF) through funding of the Systems Biology of Metabotypes grant (SysMBo 0315494A), the E-Rare project GENOMIT (01GM1207) and the German Network for mitochondrial disorders (mitoNET), including C.K., T.K. (mitoNET 01GM0862 and 01GM1113A), T.M., H.P. (mitoNET 01GM0867 and 01GM1113C) and W.S.K. (mitoNET 01GM0868). W.S.K. was funded by the Deutsche Forschungsgemeinschaft (SFB TR3 A11 and D12). V.K.M. was supported by grants from the US National Institutes of Health (GM077465 and GM097136). The financial support of Associazione Amici del Centro Dino Ferrari, University of Milan, the Telethon project GTB07001ER, the Eurobiobank project QLTR-2001-02769 and R.F. 02.187 Criobanca Automatizzata di Materiale Biologico to M.M. and M.S. are gratefully acknowledged.
Author information
Authors and Affiliations
Contributions
C.K. identified, clinically characterized, collected samples and histochemically analyzed skeletal muscle biopsies from family I and the sporadic case and obtained fibroblasts from P1976. M.S., D.R., G.P.C., M. Moggio, C.M.Q. and S.D. identified, clinically characterized, collected samples and histochemically analyzed skeletal muscle biopsies from family II and obtained fibroblasts from P4050 and P4052. T.B.H., T.W., T.M.S., T.M. and H.P. performed exome sequencing and analysis of family I. S.E.C. and V.K.M. performed targeted mitochondrial exome sequencing and analysis of family II. T.J.N., G.Z. and M. Minczuk performed the computational analysis. T.J.N. analyzed protein amounts, performed subcellular localization studies, purified and characterized recombinant MGME1 and analyzed the cells with siRNA knockdown and P1976 fibroblasts. T.B.H., K.D., A.I. and H.P. performed subcellular localization and complementation experiments. S.S. performed the mtDNA repopulation experiments. V.P. performed copy-number and deletion quantification. K.H. screened PEO samples for MGME1 mutations and identified P931. J.R. contributed to the characterization of fibroblasts from P1976. T.K. and T.M. provided samples and coordinated the German network of mitochondrial disorders. C.K., G.Z., M. Minczuk, W.S.K. and H.P. planned the project and wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Note, Supplementary Tables 1–5 and Supplementary Figures 1–8 (PDF 2064 kb)
Rights and permissions
About this article
Cite this article
Kornblum, C., Nicholls, T., Haack, T. et al. Loss-of-function mutations in MGME1 impair mtDNA replication and cause multisystemic mitochondrial disease. Nat Genet 45, 214–219 (2013). https://doi.org/10.1038/ng.2501
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng.2501
This article is cited by
-
Decoding the mitochondria without a code: mechanistic insights into mitochondrial DNA depletion syndromes
Journal of Biosciences (2024)
-
Clinical implementation of RNA sequencing for Mendelian disease diagnostics
Genome Medicine (2022)
-
POLG-related disorders and their neurological manifestations
Nature Reviews Neurology (2019)
-
Replication fork rescue in mammalian mitochondria
Scientific Reports (2019)
-
Mitochondrial dysfunction and autism: comprehensive genetic analyses of children with autism and mtDNA deletion
Behavioral and Brain Functions (2018)