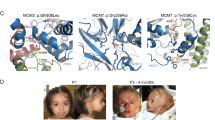
Abstract
Perlman syndrome is a congenital overgrowth syndrome inherited in an autosomal recessive manner that is associated with Wilms tumor susceptibility. We mapped a previously unknown susceptibility locus to 2q37.1 and identified germline mutations in DIS3L2, a homolog of the Schizosaccharomyces pombe dis3 gene, in individuals with Perlman syndrome. Yeast dis3 mutant strains have mitotic abnormalities. Yeast Dis3 and its human homologs, DIS3 and DIS3L1, have exoribonuclease activity and bind to the core RNA exosome complex. DIS3L2 has a different intracellular localization and lacks the PIN domain found in DIS3 and DIS3L1; nevertheless, we show that DIS3L2 has exonuclease activity. DIS3L2 inactivation was associated with mitotic abnormalities and altered expression of mitotic checkpoint proteins. DIS3L2 overexpression suppressed the growth of human cancer cell lines, and knockdown enhanced the growth of these cells. We also detected evidence of DIS3L2 mutations in sporadic Wilms tumor. These observations suggest that DIS3L2 has a critical role in RNA metabolism and is essential for the regulation of cell growth and division.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Lapunzina, P. Risk of tumorigenesis in overgrowth syndromes: a comprehensive review. Am. J. Med. Genet. C Semin. Med. Genet. 137C, 53–71 (2005).
Baujat, G. & Cormier-Daire, V. Sotos syndrome. Orphanet J. Rare Dis. 2, 36 (2007).
Alessandri, J.L. et al. Perlman syndrome: report, prenatal findings and review. Am. J. Med. Genet. A. 146A, 2532–2537 (2008).
Lim, D.H. & Maher, E.R. Genomic imprinting syndromes and cancer. Adv. Genet. 70, 145–175 (2010).
Liban, E. & Kozenitzky, I.L. Metanephric hamartomas and nephroblastomatosis in siblings. Cancer 25, 885–888 (1970).
Perlman, M., Goldberg, G.M., Bar-Ziv, J. & Danovitch, G. Renal hamartomas and nephroblastomatosis with fetal gigantism: a familial syndrome. J. Pediatr. 83, 414–418 (1973).
Perlman, M., Levin, M. & Wittels, B. Syndrome of fetal gigantism, renal hamartomas and nephroblastomatosis with Wilms' tumour. Cancer 35, 1212–1217 (1975).
Neri, G., Martini-Neri, M.E., Katz, B.E. & Opitz, J.M. The Perlman syndrome: familial renal dysplasia with Wilms tumor, fetal gigantism and multiple congenital anomalies. Am. J. Med. Genet. 19, 195–207 (1984).
Greenberg, F. et al. The Perlman familial nephroblastomatosis syndrome. Am. J. Med. Genet. 24, 101–110 (1986).
Perlman, M. Perlman syndrome: familial renal dysplasia with Wilms tumor, fetal gigantism, and multiple congenital anomalies. Am. J. Med. Genet. 25, 793–795 (1986).
Greenberg, F., Copeland, K. & Gresik, M.V. Expanding the spectrum of the Perlman syndrome. Am. J. Med. Genet. 29, 773–776 (1988).
Henneveld, H.T., van Lingen, R.A., Hamel, B.C., Stolte-Dijkstra, I. & van Essen, A.J. Perlman syndrome: four additional cases and review. Am. J. Med. Genet. 86, 439–446 (1999).
Staals, R.H. et al. Dis3-like 1: a novel exoribonuclease associated with the human exosome. EMBO J. 29, 2358–2367 (2010).
Tomecki, R. et al. The human core exosome interacts with differentially localized processive RNases: hDIS3 and hDIS3L. EMBO J. 29, 2342–2357 (2010).
Suzuki, N. et al. The Saccharomyces cerevisiae small GTPase, Gsp1p/Ran, is involved in 3′ processing of 7S-to-5.8S rRNA and in degradation of the excised 5′-A0 fragment of 35S pre-rRNA, both of which are carried out by the exosome. Genetics 158, 613–625 (2001).
Neumann, B. et al. Phenotypic profiling of the human genome by time-lapse microscopy reveals cell division genes. Nature 464, 721–727 (2010).
Fisk, H.A., Mattison, C.P. & Winey, M. Human Mps1 protein kinase is required for centrosome duplication and normal mitotic progression. Proc. Natl. Acad. Sci. USA 100, 14875–14880 (2003).
Boutros, R., Lobjois, V. & Ducommun, B. CDC25 phosphatases in cancer cells: key players? Good targets? Nat. Rev. Cancer 7, 495–507 (2007).
Kelly, A.E. & Funabiki, H. Correcting aberrant kinetochore microtubule attachments: an Aurora B-centric view. Curr. Opin. Cell Biol. 21, 51–58 (2009).
Zou, H., McGarry, T.J., Bernal, T. & Kirschner, M.W. Identification of a vertebrate sister-chromatid separation inhibitor involved in transformation and tumorigenesis. Science 285, 418–422 (1999).
Coleman, M.L., Marshall, C.J. & Olson, M.F. Ras promotes p21Waf1/Cip1 protein stability via a cyclin D1-imposed block in proteasome-mediated degradation. EMBO J. 22, 2036–2046 (2003).
Noguchi, E. et al. Dis3, implicated in mitotic control, binds directly to Ran and enhances the GEF activity of RCC1. EMBO J. 15, 5595–5605 (1996).
Shiomi, T. et al. Human dis3p, which binds to either GTP- or GDP-Ran, complements Saccharomyces cerevisiae dis3. J. Biochem. 123, 883–890 (1998).
Dziembowski, A., Lorentzen, E., Conti, E. & Séraphin, B. A single subunit, Dis3, is essentially responsible for yeast exosome core activity. Nat. Struct. Mol. Biol. 14, 15–22 (2007).
Schneider, C., Anderson, J.T. & Tollervey, D. The exosome subunit Rrp44 plays a direct role in RNA substrate recognition. Mol. Cell 27, 324–331 (2007).
Tomecki, R., Drazkowska, K. & Dziembowski, A. Mechanisms of RNA degradation by the eukaryotic exosome. ChemBioChem 11, 938–945 (2010).
Lebreton, A., Tomecki, R., Dziembowski, A. & Séraphin, B. Endonucleolytic RNA cleavage by a eukaryotic exosome. Nature 456, 993–996 (2008).
Schaeffer, D. et al. The exosome contains domains with specific endoribonuclease, exoribonuclease and cytoplasmic mRNA decay activities. Nat. Struct. Mol. Biol. 16, 56–62 (2009).
Ohkura, H. et al. Cold-sensitive and caffeine-supersensitive mutants of the Schizosaccharomyces pombe dis genes implicated in sister chromatid separation during mitosis. EMBO J. 7, 1465–1473 (1988).
Moncla, A. et al. A cluster of translocation breakpoints in 2q37 is associated with overexpression of NPPC in patients with a similar overgrowth phenotype. Hum. Mutat. 28, 1183–1188 (2007).
Wang, S.W., Stevenson, A.L., Kearsey, S.E., Watt, S. & Bähler, J. Global role for polyadenylation-assisted nuclear RNA degradation in posttranscriptional gene silencing. Mol. Cell. Biol. 28, 656–665 (2008).
Kiss, D.L. & Andrulis, E.D. Genome-wide analysis reveals distinct substrate specificities of Rrp6, Dis3, and core exosome subunits. RNA 16, 781–791 (2010).
Bocciardi, R. et al. Overexpression of the C-type natriuretic peptide (CNP) is associated with overgrowth and bone anomalies in an individual with balanced t(2;7) translocation. Hum. Mutat. 28, 724–731 (2007).
Lanktree, M.B. et al. Meta-analysis of dense genecentric association studies reveals common and uncommon variants associated with height. Am. J. Hum. Genet. 88, 6–18 (2011).
Drummond, I.A. & Davidson, A.J. Zebrafish kidney development. Methods Cell Biol. 100, 233–260 (2010).
Slanchev, K., Putz, M., Schmitt, A., Kramer-Zucker, A. & Walz, G. Nephrocystin-4 is required for pronephric duct-dependent cloaca formation in zebrafish. Hum. Mol. Genet. 20, 3119–3128 (2011).
Viot-Szoboszlai, G. et al. Wilms' tumor and gonadal dysgenesis in a child with the 2q37.1 deletion syndrome. Clin. Genet. 53, 278–280 (1998).
Conrad, B. et al. Clinical phenotype associated with terminal 2q37 deletion. Clin. Genet. 48, 134–139 (1995).
Drake, K.M. et al. Loss of heterozygosity at 2q37 in sporadic Wilms' tumor: putative role for miR-562. Clin. Cancer Res. 15, 5985–5992 (2009).
Hanks, S. et al. Constitutional aneuploidy and cancer predisposition caused by biallelic mutations in BUB1B. Nat. Genet. 36, 1159–1161 (2004).
Rio Frio, T. et al. Homozygous BUB1B mutation and susceptibility to gastrointestinal neoplasia. N. Engl. J. Med. 363, 2628–2637 (2010).
Rozen, S. & Skaletsky, H.J. Primer 3 on WWW for general users and for biologist programmers. in Bioinformatics Methods and Protocols: Methods in Molecular Biology (eds. Krawetz, S. & Misene, S.) 365–386 (Humana Press, NJ, 2000).
Schilders, G., Raijmakers, R., Raats, J.M. & Pruijn, G.J. MPP6 is an exosome-associated RNA-binding protein involved in 5.8S rRNA maturation. Nucleic Acids Res. 33, 6795–6804 (2005).
Schulte-Merker, S. Looking at embryo. in Zebrafish: A Practical Approach (eds. Nusslein-Volhard, C. & Dahm, R.) 39–58 (Oxford University Press, Oxford, 2005).
Acknowledgements
We thank J. Arrand for processing the Affymetrix 50k SNP chip. We thank the Association of International Cancer Research (07-0503) and Cancer Research UK (C485/A5441) for financial support.
Author information
Authors and Affiliations
Contributions
D.A. and M.R.M. designed, carried out and analyzed genetic and cell biological experiments; W.N.C. did initial autozygosity mapping and analysis; R.H.J.S. conducted the RNase assay; N.C.W. carried out and analyzed MLPA; H.G. did zebrafish analysis; G.A.F. and S.S. did aneuploidy studies; D.G. and C.J.R. provided technical assistance; T.C., A.J.v.E., R.A.v.L., G.N., J.M.O., P.R., I.S.-D. and E.R.M. recruited subjects, gathered clinical information and contributed clinical samples; F.M., G.J.M.P., F.L. and E.R.M. designed and supervised experiments and analyzed data; E.R.M. directed the research; E.R.M., D.A. and M.M. drafted the manuscript and all authors critically reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Table 1 and Supplementary Figures 1–11 (PDF 4311 kb)
Rights and permissions
About this article
Cite this article
Astuti, D., Morris, M., Cooper, W. et al. Germline mutations in DIS3L2 cause the Perlman syndrome of overgrowth and Wilms tumor susceptibility. Nat Genet 44, 277–284 (2012). https://doi.org/10.1038/ng.1071
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng.1071
This article is cited by
-
Hallmark discoveries in the biology of Wilms tumour
Nature Reviews Urology (2024)
-
DIS3L2 knockdown impairs key oncogenic properties of colorectal cancer cells via the mTOR signaling pathway
Cellular and Molecular Life Sciences (2023)
-
The genomic and transcriptional landscape of primary central nervous system lymphoma
Nature Communications (2022)
-
Wilms tumour
Nature Reviews Disease Primers (2021)
-
Non-coding RNAs in Wilms’ tumor: biological function, mechanism, and clinical implications
Journal of Molecular Medicine (2021)