Abstract
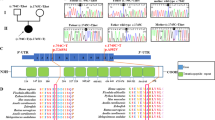
Defects in mitochondrial translation are among the most common causes of mitochondrial disease1, but the mechanisms that regulate mitochondrial translation remain largely unknown. In the yeast Saccharomyces cerevisiae, all mitochondrial mRNAs require specific translational activators, which recognize sequences in 5′ UTRs and mediate translation2. As mammalian mitochondrial mRNAs do not have significant 5′ UTRs3, alternate mechanisms must exist to promote translation. We identified a specific defect in the synthesis of the mitochondrial DNA (mtDNA)-encoded COX I subunit in a pedigree segregating late-onset Leigh syndrome and cytochrome c oxidase (COX) deficiency. We mapped the defect to chromosome 17q by functional complementation and identified a homozygous single-base-pair insertion in CCDC44, encoding a member of a large family of hypothetical proteins containing a conserved DUF28 domain. CCDC44, renamed TACO1 for translational activator of COX I, shares a notable degree of structural similarity with bacterial homologs4, and our findings suggest that it is one of a family of specific mammalian mitochondrial translational activators.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Accession codes
References
Taylor, R.W. & Turnbull, D.M. Mitochondrial DNA mutations in human disease. Nat. Rev. Genet. 6, 389–402 (2005).
Naithani, S., Saracco, S.A., Butler, C.A. & Fox, T.D. Interactions among COX1, COX2, and COX3 mRNA-specific translational activator proteins on the inner surface of the mitochondrial inner membrane of Saccharomyces cerevisiae. Mol. Biol. Cell 14, 324–333 (2003).
Montoya, J., Ojala, D. & Attardi, G. Distinctive features of the 5′-terminal sequences of the human mitochondrial mRNAs. Nature 290, 465–470 (1981).
Shin, D.H., Yokota, H., Kim, R. & Kim, S.H. Crystal structure of conserved hypothetical protein Aq1575 from Aquifex aeolicus. Proc. Natl. Acad. Sci. USA 99, 7980–7985 (2002).
Hibbs, M.A. et al. Exploring the functional landscape of gene expression: directed search of large microarray compendia. Bioinformatics 23, 2692–2699 (2007).
Fontanesi, F., Soto, I.C., Horn, D. & Barrientos, A. Assembly of mitochondrial cytochrome c-oxidase, a complicated and highly regulated cellular process. Am. J. Physiol. Cell Physiol. 291, C1129–C1147 (2006).
Mootha, V.K. et al. Identification of a gene causing human cytochrome c oxidase deficiency by integrative genomics. Proc. Natl. Acad. Sci. USA 100, 605–610 (2003).
Lurin, C. et al. Genome-wide analysis of Arabidopsis pentatricopeptide repeat proteins reveals their essential role in organelle biogenesis. Plant Cell 16, 2089–2103 (2004).
Delannoy, E., Stanley, W.A., Bond, C.S. & Small, I.D. Pentatricopeptide repeat (PPR) proteins as sequence-specificity factors in post-transcriptional processes in organelles. Biochem. Soc. Trans. 35, 1643–1647 (2007).
Xu, F., Morin, C., Mitchell, G., Ackerley, C. & Robinson, B.H. The role of the LRPPRC (leucine-rich pentatricopeptide repeat cassette) gene in cytochrome oxidase assembly: mutation causes lowered levels of COX (cytochrome c oxidase) I and COX III mRNA. Biochem. J. 382, 331–336 (2004).
Liang, H., Li, L., Dong, Z., Surette, M.G. & Duan, K. The YebC family protein PA0964 negatively regulates the Pseudomonas aeruginosa quinolone signal system and pyocyanin production. J. Bacteriol. 190, 6217–6227 (2008).
Yao, J. & Shoubridge, E.A. Expression and functional analysis of SURF1 in Leigh syndrome patients with cytochrome c oxidase deficiency. Hum. Mol. Genet. 8, 2541–2549 (1999).
Capaldi, R.A., Marusich, M.F. & Taanman, J.W. Mammalian cytochrome-c oxidase: characterization of enzyme and immunological detection of subunits in tissue extracts and whole cells. Methods Enzymol. 260, 117–132 (1995).
Antonicka, H. et al. Mutations in COX10 result in a defect in mitochondrial heme A biosynthesis and account for multiple, early-onset clinical phenotypes associated with isolated COX deficiency. Hum. Mol. Genet. 12, 2693–2702 (2003).
Klement, P., Nijtmans, L.G., Van den Bogert, C. & Houstek, J. Analysis of oxidative phosphorylation complexes in cultured human fibroblasts and amniocytes by blue-native-electrophoresis using mitoplasts isolated with the help of digitonin. Anal. Biochem. 231, 218–224 (1995).
Boulet, L., Karpati, G. & Shoubridge, E.A. Distribution and threshold expression of the tRNA(Lys) mutation in skeletal muscle of patients with myoclonic epilepsy and ragged-red fibers (MERRF). Am. J. Hum. Genet. 51, 1187–1200 (1992).
Gagnon, A., Ripeau, J.S., Zvieriev, V. & Chevrette, M. Chromosome 18 suppresses tumorigenic properties of human prostate cancer cells. Genes Chromosom. Cancer 45, 220–230 (2006).
Cuthbert, A.P. et al. Construction and characterization of a highly stable human: rodent monochromosomal hybrid panel for genetic complementation and genome mapping studies. Cytogenet. Cell Genet. 71, 68–76 (1995).
Zhu, Z. et al. SURF1, encoding a factor involved in the biogenesis of cytochrome c oxidase, is mutated in Leigh syndrome. Nat. Genet. 20, 337–343 (1998).
Antonicka, H. et al. Mutations in COX15 produce a defect in the mitochondrial heme biosynthetic pathway, causing early-onset fatal hypertrophic cardiomyopathy. Am. J. Hum. Genet. 72, 101–114 (2003).
Lochmuller, H., Johns, T. & Shoubridge, E.A. Expression of the E6 and E7 genes of human papillomavirus (HPV16) extends the life span of human myoblasts. Exp. Cell Res. 248, 186–193 (1999).
Barclay, B.J. et al. A rapid assay for mitochondrial DNA damage and respiratory chain inhibition in the yeast Saccharomyces cerevisiae. Environ. Mol. Mutagen. 38, 153–158 (2001).
Barrientos, A., Korr, D. & Tzagoloff, A. Shy1p is necessary for full expression of mitochondrial COX1 in the yeast model of Leigh's syndrome. EMBO J. 21, 43–52 (2002).
Kaufman, B.A. et al. The mitochondrial transcription factor TFAM coordinates the assembly of multiple DNA molecules into nucleoid-like structures. Mol. Biol. Cell 18, 3225–3236 (2007).
Wu, S., Skolnick, J. & Zhang, Y. Ab initio modeling of small proteins by iterative TASSER simulations. BMC Biol. 5, 17 (2007).
Zhang, Y. I-TASSER server for protein 3D structure prediction. BMC Bioinformatics 9, 40 (2008).
Zhang, Y. Template-based modeling and free modeling by I-TASSER in CASP7. Proteins 69 (Suppl 8), 108–117 (2007).
Guex, N. & Peitsch, M.C. SWISS-MODEL and the Swiss-PdbViewer: an environment for comparative protein modeling. Electrophoresis 18, 2714–2723 (1997).
Acknowledgements
We acknowledge the contribution of the individuals who cared for the study subjects and the technical assistance of I. Kaus, S. Mueller-Ziermann and A. Zimmermann. We thank T. Johns for help with immunocytochemistry and the cell culture. This work was supported in part by a grant from the Canadian Institutes of Health Research to E.A.S. E.A.S. is an International Scholar of the Howard Hughes Medical Institute. R.H. is supported by the Deutsche Forschungsgemeinschaft HO 2505/2-1.
Author information
Authors and Affiliations
Contributions
W.W. did the chromosome transfer, mutation analysis, subcellular localization and RNA immunoblotting analyses; H.A. did BN gel analyses, enzyme measurements, molecular modeling and helped write the manuscript; F.S. performed translation analyses; J.S. evaluated the index subject and affected children; B.S. evaluated the adult subjects; J.E.K. did the yeast studies; H.L. performed the linkage analysis; M.C. helped with the chromosome transfer studies; B.A.K. did the size exclusion experiments and helped with the yeast studies; R.H. performed the histological, biochemical and genetic investigation of the index subject and family members; E.A.S. designed the study and wrote the final manuscript.
Corresponding author
Supplementary information
Supplementary Text and Figures
Supplementary Note, Supplementary Table 1 and Supplementary Figures 1–7 (PDF 673 kb)
Rights and permissions
About this article
Cite this article
Weraarpachai, W., Antonicka, H., Sasarman, F. et al. Mutation in TACO1, encoding a translational activator of COX I, results in cytochrome c oxidase deficiency and late-onset Leigh syndrome. Nat Genet 41, 833–837 (2009). https://doi.org/10.1038/ng.390
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng.390
This article is cited by
-
Multi-level profiling unravels mitochondrial dysfunction in myotonic dystrophy type 2
Acta Neuropathologica (2024)
-
COX17 acetylation via MOF–KANSL complex promotes mitochondrial integrity and function
Nature Metabolism (2023)
-
Balanced mitochondrial and cytosolic translatomes underlie the biogenesis of human respiratory complexes
Genome Biology (2022)
-
Mechanisms of mitochondrial respiratory adaptation
Nature Reviews Molecular Cell Biology (2022)
-
Organization and expression of the mammalian mitochondrial genome
Nature Reviews Genetics (2022)