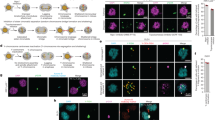
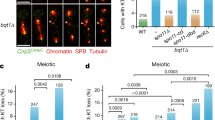
Abstract
Improper meiotic chromosome segregation causes conditions such as Down's syndrome1. Recombination promotes proper chromosome segregation in meiosis I; chromosomes without crossovers near the centromere are more likely to segregate to the same spindle pole (nondisjoin). Here we have used budding yeast to determine whether the spindle checkpoint promotes segregation of such chromosomes. In checkpoint-defective mad2Δ cells, properly segregating chromosomes have more crossovers near the centromere than their wild-type counterparts, and an artificial tether that holds chromosomes together suppresses nondisjunction as long as the tether is near the centromere. The tether partially rescues the segregation of chromosomes that lack crossovers.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Lamb, N.E., Sherman, S.L. & Hassold, T.J. Effect of meiotic recombination on the production of aneuploid gametes in humans. Cytogenet. Genome Res. 111, 250–255 (2005).
Gerton, J.L. & Hawley, R.S. Homologous chromosome interactions in meiosis: diversity amidst conservation. Nat. Rev. Genet. 6, 477–487 (2005).
Pinsky, B.A. & Biggins, S. The spindle checkpoint: tension versus attachment. Trends Cell Biol. 15, 486–493 (2005).
Nicklas, R.B. Chromosome micromanipulation. II. Induced reorientation and the experimental control of segregation in meiosis. Chromosoma 21, 17–50 (1967).
Shonn, M.A., Murray, A.L. & Murray, A.W. Spindle checkpoint component Mad2 contributes to biorientation of homologous chromosomes. Curr. Biol. 13, 1979–1984 (2003).
Cheslock, P.S., Kemp, B.J., Boumil, R.M. & Dawson, D.S. The roles of MAD1, MAD2 and MAD3 in meiotic progression and the segregation of nonexchange chromosomes. Nat. Genet. 37, 756–760 (2005).
Gilliland, W.D., Wayson, S.M. & Hawley, R.S. The meiotic defects of mutants in the Drosophila mps1 gene reveal a critical role of Mps1 in the segregation of achiasmate homologs. Curr. Biol. 15, 672–677 (2005).
Koehler, K.E. et al. Spontaneous X chromosome MI and MII nondisjunction events in Drosophila melanogaster oocytes have different recombinational histories. Nat. Genet. 14, 406–414 (1996).
Lamb, N.E. et al. Susceptible chiasmate configurations of chromosome 21 predispose to non-disjunction in both maternal meiosis I and meiosis II. Nat. Genet. 14, 400–405 (1996).
Mortimer, R.K., Schild, D., Contopoulou, C.R. & Kans, J.A. Genetic map of Saccharomyces cerevisiae, edition 10. Yeast 5, 321–403 (1989).
Kaback, D.B., Steensma, H.Y. & de Jonge, P. Enhanced meiotic recombination on the smallest chromosome of Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 86, 3694–3698 (1989).
Shonn, M.A., McCarroll, R. & Murray, A.W. Requirement of the spindle checkpoint for proper chromosome segregation in budding yeast meiosis. Science 289, 300–303 (2000).
Gu, Z. et al. Elevated evolutionary rates in the laboratory strain of Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 102, 1092–1097 (2005).
Winzeler, E.A. et al. Genetic diversity in yeast assessed with whole-genome oligonucleotide arrays. Genetics 163, 79–89 (2003).
Cherry, J.M. et al. Genetic and physical maps of Saccharomyces cerevisiae. Nature 387, 67–73 (1997).
Kramer, H. et al. lac repressor forms loops with linear DNA carrying two suitably spaced lac operators. EMBO J. 6, 1481–1491 (1987).
Chen, J. & Matthews, K.S. Subunit dissociation affects DNA binding in a dimeric lac repressor produced by C-terminal deletion. Biochemistry 33, 8728–8735 (1994).
Straight, A.F., Belmont, A.S., Robinett, C.C. & Murray, A.W. GFP tagging of budding yeast chromosomes reveals that protein-protein interactions can mediate sister chromatid cohesion. Curr. Biol. 6, 1599–1608 (1996).
Maxfield Boumil, R., Kemp, B., Angelichio, M., Nilsson-Tillgren, T. & Dawson, D.S. Meiotic segregation of a homeologous chromosome pair. Mol. Genet. Genomics 268, 750–760 (2003).
Keeney, S., Giroux, C.N. & Kleckner, N. Meiosis-specific DNA double-strand breaks are catalyzed by Spo11, a member of a widely conserved protein family. Cell 88, 375–384 (1997).
Bergerat, A. et al. An atypical topoisomerase II from Archaea with implications for meiotic recombination. Nature 386, 414–417 (1997).
Cha, R.S., Weiner, B.M., Keeney, S., Dekker, J. & Kleckner, N. Progression of meiotic DNA replication is modulated by interchromosomal interaction proteins, negatively by Spo11p and positively by Rec8p. Genes Dev. 14, 493–503 (2000).
Diaz, R.L., Alcid, A.D., Berger, J.M. & Keeney, S. Identification of residues in yeast Spo11p critical for meiotic DNA double-strand break formation. Mol. Cell. Biol. 22, 1106–1115 (2002).
Weiner, B.M. & Kleckner, N. Chromosome pairing via multiple interstitial interactions before and during meiosis in yeast. Cell 77, 977–991 (1994).
Kemp, B., Boumil, R.M., Stewart, M.N. & Dawson, D.S. A role for centromere pairing in meiotic chromosome segregation. Genes Dev. 18, 1946–1951 (2004).
Tsubouchi, T. & Roeder, G.S. A synaptonemal complex protein promotes homology-independent centromere coupling. Science 308, 870–873 (2005).
Kiburz, B.M. et al. The core centromere and Sgo1 establish a 50-kb cohesin-protected domain around centromeres during meiosis I. Genes Dev. 19, 3017–3030 (2005).
Blat, Y. & Kleckner, N. Cohesins bind to preferential sites along yeast chromosome III, with differential regulation along arms versus the centric region. Cell 98, 249–259 (1999).
Rockmill, B., Voelkel-Meiman, K. & Roeder, G.S. Centromere-proximal crossovers are associated with precocious separation of sister chromatids during meiosis in Saccharomyces cerevisiae. Genetics 174, 1745–1754 (2006).
Padmore, R., Cao, L. & Kleckner, N. Temporal comparison of recombination and synaptonemal complex formation during meiosis in S. cerevisiae. Cell 66, 1239–1256 (1991).
Acknowledgements
We thank A. Amon, D. Dawson, M. Dorer, R.S. Hawley, J. Leu, T. Salmon, F. Solomon and members of the Murray laboratory for critical reading of the manuscript; D. Dawson, J. Leu, A. Segrè and D. Thompson for discussions; and S. Keeney (Memorial Sloan-Kettering Cancer Center) and D. Dawson (Oklahoma Medical Research Foundation) for strains. This work was supported by a US National Institutes of Health (NIH) National Research Service Award fellowship to S.L. and an NIH grant to A.W.M. (GM 055840).
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Supplementary Text and Figures
Supplementary Tables 1–2 (PDF 98 kb)
Rights and permissions
About this article
Cite this article
Lacefield, S., Murray, A. The spindle checkpoint rescues the meiotic segregation of chromosomes whose crossovers are far from the centromere. Nat Genet 39, 1273–1277 (2007). https://doi.org/10.1038/ng2120
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng2120
This article is cited by
-
Maternal obesity enhances oocyte chromosome abnormalities associated with aging
Chromosoma (2019)
-
Differentiating the roles of microtubule-associated proteins at meiotic kinetochores during chromosome segregation
Chromosoma (2016)
-
De novo generation of plant centromeres at tandem repeats
Chromosoma (2013)
-
Complex regulation of sister kinetochore orientation in meiosis-I
Journal of Biosciences (2010)
-
Many functions of the meiotic cohesin
Chromosome Research (2010)