Abstract
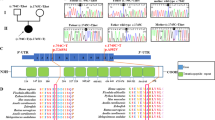
The mitochondrial (mt) DNA depletion syndromes (MDDS) are genetic disorders characterized by a severe, tissue-specific decrease of mtDNA copy number, leading to organ failure. There are two main clinical presentations: myopathic (OMIM 609560) and hepatocerebral1 (OMIM 251880). Known mutant genes, including TK2 (ref. 2), SUCLA2 (ref. 3), DGUOK (ref. 4) and POLG5,6, account for only a fraction of MDDS cases7. We found a new locus for hepatocerebral MDDS on chromosome 2p21-23 and prioritized the genes on this locus using a new integrative genomics strategy. One of the top-scoring candidates was the human ortholog of the mouse kidney disease gene Mpv17 (ref. 8). We found disease-segregating mutations in three families with hepatocerebral MDDS and demonstrated that, contrary to the alleged peroxisomal localization of the MPV17 gene product9, MPV17 is a mitochondrial inner membrane protein, and its absence or malfunction causes oxidative phosphorylation (OXPHOS) failure and mtDNA depletion, not only in affected individuals but also in Mpv17−/− mice.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Spinazzola, A. & Zeviani, M. Disorders of nuclear-mitochondrial intergenomic signaling. Gene 354, 162–168 (2005).
Saada, A. et al. Mutant mitochondrial thymidine kinase in mitochondrial DNA depletion myopathy. Nat. Genet. 29, 342–344 (2001).
Elpeleg, O. et al. Deficiency of the ADP-forming succinyl-CoA synthase activity is associated with encephalomyopathy and mitochondrial DNA depletion. Am. J. Hum. Genet. 76, 1081–1086 (2005).
Mandel, H. et al. The deoxyguanosine kinase gene is mutated in individuals with depleted hepatocerebral mitochondrial DNA. Nat. Genet. 29, 337–341 (2001).
Nguyen, K.V. et al. POLG mutations in Alpers syndrome. Neurology 65, 1493–1495 (2005).
Ferrari, G. et al. Infantile hepatocerebral syndromes associated with mutations in the mitochondrial DNA polymerase-gammaA. Brain 128, 723–731 (2005).
Salviati, L. et al. Mitochondrial DNA depletion and dGK gene mutations. Ann. Neurol. 52, 311–317 (2002).
Weiher, H., Noda, T., Gray, D.A., Sharpe, A.H. & Jaenisch, R. Transgenic mouse model of kidney disease: insertional inactivation of ubiquitously expressed gene leads to nephrotic syndrome. Cell 62, 425–434 (1990).
Zwacka, R.M. et al. The glomerulosclerosis gene Mpv17 encodes a peroxisomal protein producing reactive oxygen species. EMBO J. 13, 5129–5134 (1994).
Calvo, S., Jain, M. & Xie, X. et al. Systematic identification of human mitochondrial disease genes through integrative genomics. Nat. Genet., advance online publication 2 April 2006 (doi:10.1038/ng1776).
Trott, A. & Morano, K.A. SYM1 is the stress-induced Saccharomyces cerevisiae ortholog of the mammalian kidney disease gene Mpv17 and is required for ethanol metabolism and tolerance during heat shock. Eukaryot. Cell 3, 620–631 (2004).
Mounolou, J.C., Jakob, H. & Slonimski, P.P. Mitochondrial DNA from yeast “petite” mutants: specific changes in buoyant density corresponding to different cytoplasmic mutations. Biochem. Biophys. Res. Commun. 24, 218–224 (1966).
Tiranti, V. et al. Ethylmalonic encephalopathy is caused by mutations in ETHE1, a gene encoding a mitochondrial matrix protein. Am. J. Hum. Genet. 74, 239–252 (2004).
Koehler, C.M. New developments in mitochondrial assembly. Annu. Rev. Cell Dev. Biol. 20, 309–335 (2004).
Wiedemann, N., Frazier, A.E. & Pfanner, N. The protein import machinery of mitochondria. J. Biol. Chem. 279, 14473–14478 (2004).
Gakh, O., Cavadini, P. & Isaya, G. Mitochondrial processing peptidases. Biochim. Biophys. Acta 1592, 63–77 (2002).
Rehling, P., Pfanner, N. & Meisinger, C. Insertion of hydrophobic membrane proteins into the inner mitochondrial membrane – a guided tour. J. Mol. Biol. 326, 639–657 (2003).
Otsuka, M., Mizuno, Y., Yoshida, M., Kagawa, Y. & Ohta, S. Nucleotide sequence of cDNA encoding human cytochrome c oxidase subunit VIc. Nucleic Acids Res. 16, 10916 (1988).
Hammen, P.K., Gorenstein, D.G. & Weiner, H. Structure of the signal sequences for two mitochondrial matrix proteins that are not proteolytically processed upon import. Biochemistry 33, 8610–8617 (1994).
Meyer zum Gottesberge, A.M., Reuter, A. & Weiher, H. Inner ear defect similar to Alport's syndrome in the glomerulosclerosis mouse model Mpv17. Eur. Arch. Otorhinolaryngol. 253, 470–474 (1996).
Fernandez-Vizarra, E., Lopez-Perez, M.J. & Enriquez, J.A. Isolation of biogenetically competent mitochondria from mammalian tissues and cultured cells. Methods 26, 292–297 (2002).
Gallet, P.F. et al. Transbilayer movement and distribution of spin-labelled phospholipids in the inner mitochondrial membrane. Biochim. Biophys. Acta 1418, 61–70 (1999).
Ohba, M. & Schatz, G. Disruption of the outer membrane restores protein import to trypsin-treated yeast mitochondria. EMBO J. 6, 2117–2122 (1987).
Tiranti, V. et al. Characterization of SURF-1 expression and Surf-1p function in normal and disease conditions. Hum. Mol. Genet. 8, 2533–2540 (1999).
Fujiki, Y., Hubbard, A.L., Fowler, S. & Lazarow, P.B. Isolation of intracellular membranes by means of sodium carbonate treatment: application to endoplasmic reticulum. J. Cell. Biol. 93, 97–102 (1982).
Bugiani, M. et al. Clinical and molecular findings in children with complex I deficiency. Biochim. Biophys. Acta 1659, 136–147 (2004).
Petruzzella, V. et al. Identification and characterization of human cDNAs specific to BCS1, PET112, SCO1, COX15, and COX11, five genes involved in the formation and function of the mitochondrial respiratory chain. Genomics 54, 494–504 (1998).
Ho, S.N., Hunt, H.D., Horton, R.M., Pullen, J.K. & Pease, L.R. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene 77, 51–59 (1989).
Fontanesi, F. et al. Mutations in AAC2, equivalent to human adPEO-associated ANT1 mutations, lead to defective oxidative phosphorylation in Saccharomyces cerevisiae and affect mitochondrial DNA stability. Hum. Mol. Genet. 13, 923–934 (2004).
Winterthun, S. et al. Autosomal recessive mitochondrial ataxic syndrome due to mitochondrial polymerase gamma mutations. Neurology 64, 1204–1208 (2005).
Acknowledgements
We are indebted to B. Geehan for revising the manuscript, E. Lamantea for technical assistance and L. Palmieri for critical discussion. This work was supported by Fondazione Telethon-Italy (grant GGP030039), Fondazione Pierfranco e Luisa Mariani and MITOCIRCLE and EUMITOCOMBAT network grants from the European Union Framework Program 6.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
Clinical and molecular characterization of individuals with mutant MPV17. (PDF 101 kb)
Supplementary Fig. 2
Alkali treatment of mitochondrial membranes. (PDF 87 kb)
Supplementary Table 1
Strategy for site-directed mutagenesis. (PDF 40 kb)
Supplementary Table 2
Oligonucleotides used for DNA blot and real-time PCR analyses on mouse genome. (PDF 32 kb)
Supplementary Table 3
Strategy for nucleotide sequencing of the human MPV17 gene. (PDF 31 kb)
Rights and permissions
About this article
Cite this article
Spinazzola, A., Viscomi, C., Fernandez-Vizarra, E. et al. MPV17 encodes an inner mitochondrial membrane protein and is mutated in infantile hepatic mitochondrial DNA depletion. Nat Genet 38, 570–575 (2006). https://doi.org/10.1038/ng1765
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng1765
This article is cited by
-
Revealing mitf functions and visualizing allografted tumor metastasis in colorless and immunodeficient Xenopus tropicalis
Communications Biology (2024)
-
A Drosophila model of the neurological symptoms in Mpv17-related diseases
Scientific Reports (2022)
-
PyMPV17, the MPV17 Homolog of Pyropia yezoensis (Rhodophyta), Enhances Osmotic Stress Tolerance in Chlamydomonas
Plant Molecular Biology Reporter (2020)
-
Super-resolution quantification of nanoscale damage to mitochondria in live cells
Nano Research (2020)
-
Clinical and molecular characterization of three patients with Hepatocerebral form of mitochondrial DNA depletion syndrome: a case series
BMC Medical Genetics (2019)