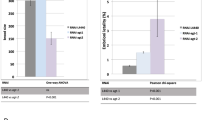
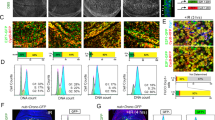
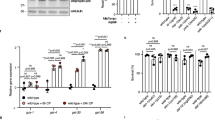
Abstract
c-Abl, a conserved nonreceptor tyrosine kinase, integrates genotoxic stress responses, acting as a transducer of both pro- and antiapoptotic effector pathways1. Nuclear c-Abl seems to interact with the p53 homolog p73 to elicit apoptosis2,3. Although several observations suggest that cytoplasmic localization of c-Abl is required for antiapoptotic function4,5, the signals that mediate its antiapoptotic effect are largely unknown. Here we show that worms carrying an abl-1 deletion allele, abl-1(ok171), are specifically hypersensitive to radiation-induced apoptosis in the Caenorhabditis elegans germ line. Our findings delineate an apoptotic pathway antagonized by ABL-1, which requires sequentially the cell cycle checkpoint genes clk-2, hus-1 and mrt-2; the C. elegans p53 homolog, cep-1; and the genes encoding the components of the conserved apoptotic machinery, ced-3, ced-9 and egl-1. ABL-1 does not antagonize germline apoptosis induced by the DNA-alkylating agent ethylnitrosourea. Furthermore, worms treated with the c-Abl inhibitor STI-571 (Gleevec; used in human cancer therapy), two newly synthesized STI-571 variants or PD166326 had a phenotype similar to that generated by abl-1(ok171). These studies indicate that ABL-1 distinguishes proapoptotic signals triggered by two different DNA-damaging agents and suggest that C. elegans might provide tissue models for development of anticancer drugs.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Wang, J.Y. Regulation of cell death by the Abl tyrosine kinase. Oncogene 19, 5643–5650 (2000).
Gong, J.G. et al. The tyrosine kinase c-Abl regulates p73 in apoptotic response to cisplatin-induced DNA damage. Nature 399, 806–809 (1999).
Agami, R., Blandino, G., Oren, M. & Shaul, Y. Interaction of c-Abl and p73alpha and their collaboration to induce apoptosis. Nature 399, 809–813 (1999).
Vigneri, P. & Wang, J.Y. Induction of apoptosis in chronic myelogenous leukemia cells through nuclear entrapment of BCR-ABL tyrosine kinase. Nat. Med. 7, 228–234 (2001).
Barila, D. et al. Caspase-dependent cleavage of c-Abl contributes to apoptosis. Mol. Cell. Biol. 23, 2790–2799 (2003).
Wen, S.T., Jackson, P.K. & Van Etten, R.A. The cytostatic function of c-Abl is controlled by multiple nuclear localization signals and requires the p53 and Rb tumor suppressor gene products. EMBO J. 15, 1583–1595 (1996).
la Cour, T. et al. NESbase version 1.0: a database of nuclear export signals. Nucleic Acids Res. 31, 393–396 (2003).
Ellis, R.E., Jacobson, D.M. & Horvitz, H.R. Genes required for the engulfment of cell corpses during programmed cell death in Caenorhabditis elegans. Genetics 129, 79–94 (1991).
Zhou, Z., Caron, E., Hartwieg, E., Hall, A. & Horvitz, H.R. The C. elegans PH domain protein CED-12 regulates cytoskeletal reorganization via a Rho/Rac GTPase signaling pathway. Dev. Cell 1, 477–489 (2001).
Krause, M., Harrison, S.W., Xu, S.Q., Chen, L. & Fire, A. Elements regulating cell- and stage-specific expression of the C. elegans MyoD family homolog hlh-1. Dev. Biol. 166, 133–148 (1994).
Hsieh, J. et al. The RING finger/B-box factor TAM-1 and a retinoblastoma-like protein LIN-35 modulate context-dependent gene silencing in Caenorhabditis elegans. Genes Dev. 13, 2958–2970 (1999).
Hengartner, M.O., Ellis, R.E. & Horvitz, H.R. Caenorhabditis elegans gene ced-9 protects cells from programmed cell death. Nature 356, 494–499 (1992).
Shaham, S., Reddien, P.W., Davies, B. & Horvitz, H.R. Mutational analysis of the Caenorhabditis elegans cell-death gene ced-3. Genetics 153, 1655–1671 (1999).
Gartner, A., Milstein, S., Ahmed, S., Hodgkin, J. & Hengartner, M.O. A conserved checkpoint pathway mediates DNA damage-induced apoptosis and cell cycle arrest in C. elegans. Mol. Cell 5, 435–443 (2000).
Chin, G.M. & Villeneuve, A.M. C. elegans mre-11 is required for meiotic recombination and DNA repair but is dispensable for the meiotic G(2) DNA damage checkpoint. Genes Dev. 15, 522–534 (2001).
Takanami, T., Mori, A., Takahashi, H. & Higashitani, A. Hyper-resistance of meiotic cells to radiation due to a strong expression of a single recA-like gene in Caenorhabditis elegans. Nucleic Acids Res. 28, 4232–4236 (2000).
Boulton, S.J. et al. Combined functional genomic maps of the C. elegans DNA damage response. Science 295, 127–131 (2002).
Rinaldo, C., Bazzicalupo, P., Ederle, S., Hilliard, M. & La Volpe, A. Roles for Caenorhabditis elegans rad-51 in meiosis and in resistance to ionizing radiation during development. Genetics 160, 471–479 (2002).
Derry, W.B., Putzke, A.P. & Rothman, J.H. Caenorhabditis elegans p53: role in apoptosis, meiosis, and stress resistance. Science 294, 591–595 (2001).
Schumacher, B., Hofmann, K., Boulton, S. & Gartner, A. The C. elegans homolog of the p53 tumor suppressor is required for DNA damage-induced apoptosis. Curr. Biol. 11, 1722–1727 (2001).
Kurzrock, R., Gutterman, J.U. & Talpaz, M. The molecular genetics of Philadelphia chromosome-positive leukemias. N. Engl. J. Med. 319, 990–998 (1988).
Druker, B.J. STI571 (Gleevec) as a paradigm for cancer therapy. Trends Mol. Med. 8, S14–S18 (2002).
Nagar, B. et al. Crystal structures of the kinase domain of c-Abl in complex with the small molecule inhibitors PD173955 and imatinib (STI-571). Cancer Res. 62, 4236–4243 (2002).
Gumienny, T.L., Lambie, E., Hartwieg, E., Horvitz, H.R. & Hengartner, M.O. Genetic control of programmed cell death in the Caenorhabditis elegans hermaphrodite germline. Development 126, 1011–1022 (1999).
Fire, A. et al. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 391, 806–811 (1998).
Buchdunger, E. et al. Inhibition of the Abl protein-tyrosine kinase in vitro and in vivo by a 2-phenylaminopyrimidine derivative. Cancer Res. 56, 100–104 (1996).
Hamby, J.M. et al. Structure-activity relationships for a novel series of pyrido[2,3-d]pyrimidine tyrosine kinase inhibitors. J. Med. Chem. 40, 2296–2303 (1997).
Berman, E. et al. Characterization of two novel sublines established from a human megakaryoblastic leukemia cell line transfected with p210(BCR-ABL). Leuk. Res. 24, 289–297 (2000).
Wisniewski, D. et al. Characterization of potent inhibitors of the Bcr-Abl and the c-kit receptor tyrosine kinases. Cancer Res. 62, 4244–4255 (2002).
Hofmann, E.R. et al. Caenorhabditis elegans HUS-1 is a DNA damage checkpoint protein required for genome stability and EGL-1–mediated apoptosis. Curr. Biol. 12, 1908–1918 (2002).
Acknowledgements
We thank G. Garriga for the strain gmEx223[pabl-1:GFP], S. Lowe for the retrovirus vectors pLPC and pLPC-h-p53, J. Wang and S. Shaham for critical reading of the manuscript and H. Xing, X. Lin, H, Lee and J. Zhang for technical assistance. This work was supported by grants from the National Institutes of Health to R.K., Z.F., M.O.H., E.R.H. and O.H. and from the Norman and Rosita Winston Foundation to X.D. A.V. is funded by the Spanish Ministry of Science and Technology.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
abl-1 encodes five different transcripts generated by cis- and or trans-splicing mechanisms that yield different NH2-termini. (PDF 51 kb)
Supplementary Fig. 2
36 hours post 120 Gy irradiation, germ cell apoptosis in wild-type worms was detected with SYTO as viewed by Nomarski DIC optics and fluorescence imaging. (PDF 361 kb)
Supplementary Fig. 3
The interaction of ABL-1 and human p53 in HEK293 cells after ionizing radiation. (PDF 34 kb)
Supplementary Fig. 4
The schematic diagram of the interaction of PD166326 with the kinase domains of human Abl and C. elegans ABL-1. (PDF 83 kb)
Supplementary Table 1
Germ cell corpses are removed at a normal rate in abl-1(ok171). (PDF 3 kb)
Supplementary Table 2
Programmed cell death genes are required for radiation-induced germ cell apoptosis in abl-1(ok171). (PDF 3 kb)
Rights and permissions
About this article
Cite this article
Deng, X., Hofmann, E., Villanueva, A. et al. Caenorhabditis elegans ABL-1 antagonizes p53-mediated germline apoptosis after ionizing irradiation. Nat Genet 36, 906–912 (2004). https://doi.org/10.1038/ng1396
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng1396
This article is cited by
-
The ABL-MYC axis controls WIPI1-enhanced autophagy in lifespan extension
Communications Biology (2023)
-
Sensitive Fibre-Based Thermoluminescence Detectors for High Resolution In-Vivo Dosimetry
Scientific Reports (2015)
-
c-Abl and Arg tyrosine kinases regulate lysosomal degradation of the oncoprotein Galectin-3
Cell Death & Differentiation (2010)
-
DNA damage stress response in germ cells: role of c-Abl and clinical implications
Oncogene (2010)
-
Delayed activation of Bax by DNA damage in embryonic stem cells with knock-in mutations of the Abl nuclear localization signals
Cell Death & Differentiation (2007)