Abstract
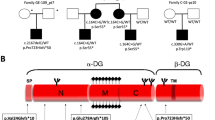
Desmin-related myopathies (DRM) are inherited neuromuscular disorders characterized by adult onset and delayed accumulation of aggregates of desmin, a protein belonging to the type III intermediate filament family, in the sarcoplasma of skeletal and cardiac muscles1,2. In this paper, we have mapped the locus for DRM in a large French pedigree to a 26-cM interval in chromosome 11q21–23. This region contains the αB-crystallin gene (CRYAB), a candidate gene encoding a 20-kD protein that is abundant in lens and is also present in a number of non-ocular tissues, including cardiac and skeletal muscle3,4. αB-crystallin is a member of the small heat shock protein (shsp) family and possesses molecular chaperone activity5. We identified an R120G missense mutation in CRYAB that co-segregates with the disease phenotype in this family. Muscle cell lines transfected with the mutant CRYAB cDNA showed intracellular aggregates that contain both desmin and αB-crystallin as observed in muscle fibers from DRM patients. These results are the first to identify a defect in a molecular chaperone as a cause for an inherited human muscle disorder.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Accession codes
References
Goebel, H. & Fardeau, M. Familial Desmin-related myopathies and cardiomyopathies from myopathology to molecular and clinical genetics. Neuromuscul. Disord. 6, 383–388 (1996).
Fuchs, E. & Cleveland, D. A structural scaffolding of intermediate filaments in health and disease. Science 279, 514–519 (1998).
Bhat, S. & Nagineni, C. αB subunit of lens-specific protein α-crystallin is present in other ocular and nonocular tissue. Biochem. Biophys. Res. Commun. 158, 319–325 (1989).
Dubin, R., Wawrousek, E. & Piatigorsky, J. Expression of the murine αB-crystallin is not restricted to the lens. Mol. Cell Biol. 9, 1083–1091 (1989).
Graw, J. The crystallins: genes, proteins and diseases. Biol. Chem. 378, 1331–1348 (1997).
Fardeau, M. et al. Une nouvelle affection musculaire familiale définie par l’accumulation intra-sarcoplasmique d’un matériel granulofilamentaire dense en microscopie electronique. Rev. Neurol. (Paris) 134, 411–425 (1978).
Rappaport, L. et al. Storage of phosphorylated desmin in a familial myopathy. FEBS Lett. 231, 421–425 (1988).
Vicart, P. et al. Human desmin gene: cDNA sequence, regional localization and exclusion of the locus in a familial desmin-related myopathy. Hum. Genet. 98, 422–429 (1996).
JeanPierre, C., Austry, E., Delattre, O., Jones, C. & Junien, C. Subregional physical mapping of an αβ-crystallin sequence and of a new expressed sequence D11S877E to human 11q. Mamm. Genome 4, 104–108 (1993).
Collins, A., Frézal, J., Teague, J. & Morton, N.E. A metric map of humans: 23,500 loci in 850 bands. Proc. Natl Acad. Sci. USA 93, 14771–14775 (1996).
Li, Z. et al. Desmin is essential for the tensile strength and integrity of myofibrils but not for myogenic commitment, differentiation, and fusion of skeletal muscle. J. Cell Biol. 139, 129–144 (1997).
Bennardini, F., Wrzosck, A. & Chiesi, M. αB-crystallin in cardiac tissue: association with actin and desmin filaments. Circ. Res. 71, 288–294 (1992).
Djabali, K., deNéchaud, B., Landon, F. & Portier, M. αB-crystallin interacts with intermediate filaments in response to stress. J. Cell Sci. 110, 2759–2769 (1997).
Liao, J., Hung, C., Lee, J., Wu, S. & Chiou, S. Characterization, cloning, and expression of porcine alpha-B crystallin. Biochem. Biophys. Res. Commun. 244, 131–137 (1998).
Suzuki, A. et al. MKBP, a novel member of the small heat shock protein family, binds and activates the myotonic dystrophy protein kinase. J. Cell Biol. 140, 1113–1124 (1998).
Litt, M. et al. Autosomal dominant congenital cataract associated with a missense mutation in the human alpha crystallin gene CRYAA. Hum. Mol. Genet. 7, 471–474 (1998).
Martin, J., Mestril, R., Hilaldandan, R., Brunton, L. & Dillmann, W. Small heat shock proteins and protection against ischemic injury in cardiac myocytes. Circulation 96, 4343–4348 (1997).
Vandeklundert, F., Gijsen, M., Vandenijssel, P., Snoeckx, L. & Dejong, W. Alpha-B-crystallin and Hsp25 in neonatal cardiac cells. Differences in cellular localization under stress conditions. Eur. J. Cell Biol. 75, 38–45 (1998).
Neufer, P., Ordway, G. & Williams, R. Transient regulation of c-fos, α-B-crystallin, and hsp70 in muscle during recovery from contractile activity. Am. J. Physiol. 43, C341–C346 (1998).
Lobrinus, J.A. et al. Familial cardiomyopathy and distal myopathy with an abnormal desmin accumulation and migration. Neuromuscul. Disord. 8, 77–86 (1998).
Bertini, E. et al. Neuropathy and restrictive cardiomyopathy with accumulation of intermediate filaments: a clinical, morphological and biochemical study. Acta Neuropathol. 81, 632–640 (1991).
Baeta, A., Figarella-Branger, D., Bille-Turc, F., Lepidi, H. & Pellissier, J.-F. Familial desmin myopathies and cytoplasmic body myopathies. Acta Neuropathol 92, 499–510 (1996).
Horowitz, S. & Schmalbruch, H. Autosomal dominant distal myopathy with desmin storage: a clinicopathologic and electrophysiologic study of a large kinship. Muscle Nerve 17, 151–160 (1994).
Iwaki, T., Kume-Iwaki, A., Liem, R. & Goldman, J. αB-crystallin is expressed in non-lenticular tissues and accumulates in Alexander's disease brain. Cell 57, 71–78 (1989).
Kato, S. et al. Pathological characterization of astrocytic hyaline inclusions in familial amyotrophic lateral sclerosis. Am. J. Pathol. 151, 611–620 (1997).
Dib, C. et al. A comprehensive genetic map of the human genome based on 5,264 microsatellites. Nature 14, 152–154 (1996).
Gyapay, G., Ginot, F., Nguyen, S., Vignal, A. & Weissenbach, J. Genotyping procedures in linkage mapping. Meth. Enzymol. 9, 91–97 (1996).
Vignal, A. et al. Non-radioactive multiplex procedure for genotyping of microsatellite markers. in Meth. Mol. Genet. (ed. Adolph, K.W.) 211–221 (Academic Press, New York, 1993).
Helbling-Leclerc, A. et al. Mutations in the laminin α 2-chain gene (LAMA2) cause merosin- deficient congenital muscular dystrophy. Nature Genet. 11, 216–218 (1995).
Tilney, L. & Portnoy, D. Actin filaments and the growth, movement and spread of the intracellular bacterial parasite, Listeria monocytogenes. J. Cell Biol. 109, 1597–1608 (1989).
Acknowledgements
We thank the family members for their help with this study. We also acknowledge R. Hellio for assistance with confocal microscopy. We thank M.M. Portier, G. Butler-Browne and R. Krishnamoorthy for helpful discussion. This work was supported by Association Française contre les Myopathies and Ministère de l’Education Nationale, de l'Enseignement Supérieur et de la Recherche.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vicart, P., Caron, A., Guicheney, P. et al. A missense mutation in the αB-crystallin chaperone gene causes a desmin-related myopathy. Nat Genet 20, 92–95 (1998). https://doi.org/10.1038/1765
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/1765
This article is cited by
-
Hallmarks of cardiovascular ageing
Nature Reviews Cardiology (2023)
-
Distinctive chaperonopathy in skeletal muscle associated with the dominant variant in DNAJB4
Acta Neuropathologica (2023)
-
Critical functions of extracellular matrix in brain metastasis seeding
Cellular and Molecular Life Sciences (2023)
-
Loss of function variants in DNAJB4 cause a myopathy with early respiratory failure
Acta Neuropathologica (2023)
-
Molecular and cellular basis of genetically inherited skeletal muscle disorders
Nature Reviews Molecular Cell Biology (2021)