Abstract
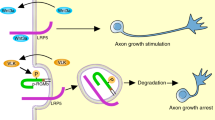
How a neuron becomes polarized remains largely unknown. Results obtained with a function-blocking antibody and an siRNA targeting the insulin-like growth factor-1 (IGF-1) receptor suggest that an essential step in the establishment of hippocampal neuronal polarity and the initiation of axonal outgrowth is the activation of the phosphatidylinositol 3-kinase (PI3k)-Cdc42 pathway by the IGF-1 receptor, but not by the TrkA or TrkB receptors.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Craig, A.M. & Banker, G. Annu. Rev. Neurosci. 17, 267–310 (1994).
Shi, S.H., Jan, L.Y. & Jan, Y.N. Cell 112, 63–75 (2003).
Ménager, C., Arimura, N., Fukata, Y. & Kaibuchi, K. J. Neurochem. 89, 109–118 (2004).
Nishimura, T. et al. Nat. Cell Biol. 7, 270–277 (2005).
Quiroga, S., Garofalo, R. & Pfenninger, K.H. Proc. Natl. Acad. Sci. USA 92, 4309–4312 (1995).
Mascotti, F., Cáceres, A., Pfenninger, K.H. & Quiroga, S. J. Neurosci. 17, 1447–1459 (1997).
Schwamborn, J.C. & Puschel, A.W. Nat. Neurosci. 7, 923–929 (2004).
Roudabush, F.L., Pierce, K.L., Maudsley, S., Khan, K.D. & Luttrell, L.M. J. Biol. Chem. 275, 22583–22589 (2000).
Da Silva, J.S., Hasegawa, T., Miyagi, T., Dotti, C.G. & Abad-Rodriguez, J. Nat. Neurosci. 8, 606–615 (2005).
Smeyne, R.J. et al. Nature 368, 246–249 (1994).
Liu, J.P., Baker, J., Perkins, A.S., Robertson, E.J. & Efstratiadis, A. Cell 75, 59–72 (1993).
Zheng, W.H. & Quirion, R. J. Neurochem. 89, 844–852 (2004).
Pfenninger, K.H. et al. J. Cell Sci. 116, 1209–1217 (2003).
Laurino, L. et al. J. Cell Sci. 118, 3653–3663 (2005).
Klein, R. et al. Cell 75, 113–122 (1993).
Acknowledgements
This work was supported by grants from Consejo de Investigaciones Científicas y Técnicas (CONICET), Agencia Córdoba Ciencia, Secretaría de Ciencia y Tecnología de la Universidad Nacional de Córdoba (SECYT-U.N.C.) and Ministerio de Salud, República Argentina (to S.Q.); by the US National Institutes of Health (grant R01 NS41029 to K.H.P.); by a Fogarty International Research Collaboration Award (1R03 TW05763 to K.H.P. and S.Q.); by the Agencia Nacional de Promoción Científica y Tecnológica, Argentina (A.C., S.Q. and G.P.); and by the International Research Scholar Program of the Howard Hughes Medical Institute (HHMI 75197-553201 to A.C.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
Specificity of the anti-active IGF-1R antibody. (PDF 281 kb)
Supplementary Fig. 2
Tau did not distribute to a single neurite in αIR3 treated neurons. (PDF 296 kb)
Supplementary Fig. 3
Transfection with an IGF-1R targeted siRNA silenced the expression of βgc-containing IGF-1R to non-detectable levels and inhibited hippocampal neuron polarization. (PDF 230 kb)
Supplementary Fig. 4
siRNA-treated neurons showed no significant differences in viability, whereas significant differences were evident in neuronal polarization. (PDF 351 kb)
Supplementary Fig. 5
siRNA treated neurons expressed near-normal levels of activatable BDNF receptor but were unable to form axons. (PDF 327 kb)
Supplementary Fig. 6
Neurons expressed insulin receptor (not affected by IGF-1R-targeted siRNA). (PDF 278 kb)
Supplementary Fig. 7
Co-transfection with a dominant-negative form of cdc-42 did not produce any noticeable effects in the IGF-1R-suppressed neurons. (PDF 235 kb)
Rights and permissions
About this article
Cite this article
Sosa, L., Dupraz, S., Laurino, L. et al. IGF-1 receptor is essential for the establishment of hippocampal neuronal polarity. Nat Neurosci 9, 993–995 (2006). https://doi.org/10.1038/nn1742
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn1742
This article is cited by
-
The neurobiology of insulin-like growth factor I: From neuroprotection to modulation of brain states
Molecular Psychiatry (2023)
-
IGFBPL1 Regulates Axon Growth through IGF-1-mediated Signaling Cascades
Scientific Reports (2018)
-
PAR3–PAR6–atypical PKC polarity complex proteins in neuronal polarization
Cellular and Molecular Life Sciences (2018)
-
Discovery of long-range inhibitory signaling to ensure single axon formation
Nature Communications (2017)
-
IGF-1 receptor regulates dynamic changes in neuronal polarity during cerebral cortical migration
Scientific Reports (2017)