Abstract
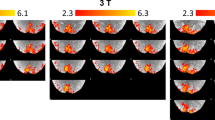
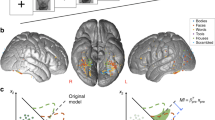
Most functional brain imaging studies use task-induced hemodynamic responses to infer underlying changes in neuronal activity. In addition to increases in cerebral blood flow and blood oxygenation level–dependent (BOLD) signals, sustained negative responses are pervasive in functional imaging. The origin of negative responses and their relationship to neural activity remain poorly understood. Through simultaneous functional magnetic resonance imaging and electrophysiological recording, we demonstrate a negative BOLD response (NBR) beyond the stimulated regions of visual cortex, associated with local decreases in neuronal activity below spontaneous activity, detected 7.15 ± 3.14 mm away from the closest positively responding region in V1. Trial-by-trial amplitude fluctuations revealed tight coupling between the NBR and neuronal activity decreases. The NBR was associated with comparable decreases in local field potentials and multiunit activity. Our findings indicate that a significant component of the NBR originates in neuronal activity decreases.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Raichle, M.E., Martin, W.R.W., Herscovitch, P., Mintun, M.A. & Markham, J. Brain blood-flow measured with intravenous H2(15)O. II. Implementation and validation. J. Nucl. Med. 24, 790–798 (1983).
Ogawa, S., Lee, T.M., Kay, A.R. & Tank, D.W. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc. Natl. Acad. Sci. USA 87, 9868–9872 (1990).
Kwong, K.K. et al. Dynamic magnetic resonance imaging of human brain activity during primary sensory stimulation. Proc. Natl. Acad. Sci. USA 89, 5675–5679 (1992).
Ogawa, S. et al. Intrinsic signal changes accompanying sensory stimulation: functional brain mapping with magnetic-resonance-imaging. Proc. Natl. Acad. Sci. USA 89, 5951–5955 (1992).
Bandettini, P.A., Wong, E.C., Hinks, R.S., Tikofsky, R.S. & Hyde, J.S. Time course EPI of human brain function during task activation. Magn. Reson. Med. 25, 390–397 (1992).
Mathiesen, C., Caesar, K., Akgoren, N. & Lauritzen, M. Modification of activity-dependent increases of cerebral blood flow by excitatory synaptic activity and spikes in rat cerebellar cortex. J. Physiol. (Lond.) 512, 555–566 (1998).
Logothetis, N.K., Pauls, J., Augath, M., Trinath, T. & Oeltermann, A. Neurophysiological investigation of the basis of the fMRI signal. Nature 412, 150–157 (2001).
Smith, A.J. et al. Cerebral energetics and spiking frequency: the neurophysiological basis of fMRI. Proc. Natl. Acad. Sci. USA 99, 10765–10770 (2002).
Sheth, S. et al. Evaluation of coupling between optical intrinsic signals and neuronal activity in rat somatosensory cortex. Neuroimage 19, 884–894 (2003).
Thompson, J.K., Peterson, M.R. & Freeman, R.D. Single-neuron activity and tissue oxygenation in the cerebral cortex. Science 299, 1070–1072 (2003).
Devor, A. et al. Coupling of total hemoglobin concentration, oxygenation, and neural activity in rat somatosensory cortex. Neuron 39, 353–359 (2003).
Kayser, C., Kim, M., Ugurbil, K., Kim, D.S. & Konig, P. A comparison of hemodynamic and neural responses in cat visual cortex using complex stimuli. Cereb. Cortex 14, 881–891 (2004).
Niessing, J. et al. Hemodynamic signals correlate tightly with synchronized gamma oscillations. Science 309, 948–951 (2005).
Mukamel, R. et al. Coupling between neuronal firing, field potentials, and fMR1 in human auditory cortex. Science 309, 951–954 (2005).
Shulman, G.L. et al. Common blood flow changes across visual tasks: II. Decreases in cerebral cortex. J. Cogn. Neurosci. 9, 648–663 (1997).
Shmuel, A. et al. Sustained negative BOLD, blood flow and oxygen consumption response and its coupling to the positive response in the human brain. Neuron 36, 1195–1210 (2002).
Logothetis, N.K. The neural basis of the blood-oxygen-level-dependent functional magnetic resonance imaging signal. Phil. Trans. R. Soc. Lond. B 357, 1003–1037 (2002).
Tootell, R.B.H. et al. The retinotopy of visual spatial attention. Neuron 21, 1409–1422 (1998).
Saad, Z.S., Ropella, K.M., Cox, R.W. & Deyoe, E.A. Analysis and use of fMRI response delays. Hum. Brain Mapp. 13, 74–93 (2001).
Harel, N., Lee, S.P., Nagaoka, T., Kim, D.S. & Kim, S.G. Origin of negative blood oxygenation level-dependent fMRI signals. J. Cereb. Blood Flow Metab. 22, 908–917 (2002).
Kannurpatti, S.S. & Biswal, B.B. Negative functional response to sensory stimulation and its origins. J. Cereb. Blood Flow Metab. 24, 703–712 (2004).
Devor, A. et al. Coupling of the cortical hemodynamic response to cortical and thalamic neuronal activity. Proc. Natl. Acad. Sci. USA 102, 3822–3827 (2005).
Allison, J.D., Meader, K.J., Loring, D.W., Figueroa, R.E. & Wright, J.C. Functional MRI cerebral activation and deactivation during finger movement. Neurology 54, 135–142 (2000).
Raichle, M.E. et al. A default mode of brain function. Proc. Natl. Acad. Sci. USA 98, 676–682 (2001).
Gusnard, D.A. & Raichle, M.E. Searching for a baseline: functional imaging and the resting human brain. Nat. Rev. Neurosci. 2, 685–694 (2001).
Smith, A.T., Williams, A.L. & Singh, K.D. Negative BOLD in the visual cortex: evidence against blood stealing. Hum. Brain Mapp. 21, 213–220 (2004).
Gold, L. & Lauritzen, M. Neuronal deactivation explains decreased cerebellar blood flow in response to focal cerebral ischemia or suppressed neocortical function. Proc. Natl. Acad. Sci. USA 99, 7699–7704 (2002).
Stefanovic, B., Warnking, J.M. & Pike, G.B. Hemodynamic and metabolic responses to neuronal inhibition. Neuroimage 22, 771–778 (2004).
Gilbert, C.D. & Wiesel, T.N. Morphology and intra-cortical projections of functionally characterized neurons in the cat visual cortex. Nature 280, 120–125 (1979).
Rockland, K.S. & Lund, J.S. Intrinsic laminar lattice connections in primate visual cortex. J. Comp. Neurol. 216, 303–318 (1983).
Pfeuffer, J., Merkle, H., Beyerlein, M., Steudel, T. & Logothetis, N.K. Anatomical and functional MR imaging in the macaque monkey using a vertical large-bore 7 Tesla setup. Magn. Reson. Imaging 22, 1343–1359 (2004).
Mulligan, S.J. & MacVicar, B.A. Calcium transients in astrocyte endfeet cause cerebrovascular constrictions. Nature 431, 195–199 (2004).
Cauli, B. et al. Cortical GABA interneurons in neurovascular coupling: relays for subcortical vasoactive pathways. J. Neurosci. 24, 8940–8949 (2004).
Hoge, R.D. et al. Investigation of BOLD signal dependence on cerebral blood flow and oxygen consumption: the deoxyhemoglobin dilution model. Magn. Reson. Med. 42, 849–863 (1999).
Buxton, R.B., Uludag, K., Dubowitz, D.J. & Liu, T.T. Modeling the hemodynamic response to brain activation. Neuroimage 23, S220–S233 (2004).
Lauritzen, M. Reading vascular changes in brain imaging: is dendritic calcium the key? Nat. Rev. Neurosci. 6, 77–85 (2005).
Malonek, D. & Grinvald, A. Interactions between electrical activity and cortical microcirculation revealed by imaging spectroscopy: implications for functional brain mapping. Science 272, 551–554 (1996).
Menon, R.S. et al. Bold based functional MRI at 4-tesla includes a capillary bed contribution: echo-planar imaging correlates with previous optical imaging using intrinsic signals. Magn. Reson. Med. 33, 453–459 (1995).
Logothetis, N.K., Guggenberger, H., Peled, S. & Pauls, J. Functional imaging of the monkey brain. Nat. Neurosci. 2, 555–562 (1999).
Uludag, K. et al. Coupling of cerebral blood flow and oxygen consumption during physiological activation and deactivation measured with fMRI. Neuroimage 23, 148–155 (2004).
Iadecola, C. Neurovascular regulation in the normal brain and in Alzheimer's disease. Nat. Rev. Neurosci. 5, 347–360 (2004).
Duvernoy, H.M., Delon, S. & Vannson, J.L. Cortical blood vessels of the human-brain. Brain Res. Bull. 7, 519–579 (1981).
Logothetis, N.K. The underpinnings of the BOLD functional magnetic resonance imaging signal. J. Neurosci. 23, 3963–3971 (2003).
Mandeville, J.B. et al. Dynamic functional imaging of relative cerebral blood volume during rat forepaw stimulation. Magn. Reson. Med. 39, 615–624 (1998).
Buxton, R.B., Wong, E.C. & Frank, L.R. Dynamics of blood flow and oxygenation changes during brain activation: the balloon model. Magn. Reson. Med. 39, 855–864 (1998).
Lu, H., Golay, X., Pekar, J.J. & van Zijl, P.C.M. Sustained poststimulus elevation in cerebral oxygen utilization after vascular recovery. J. Cereb. Blood Flow Metab. 24, 764–770 (2004).
Brewer, A.A., Press, W.A., Logothetis, N.K. & Wandell, B.A. Visual areas in macaque cortex measured using functional magnetic resonance imaging. J. Neurosci. 22, 10416–10426 (2002).
Bandettini, P.A., Jesmanowicz, A., Wong, E.C. & Hyde, J.S. Processing strategies for time-course data sets in functional MRI of the human brain. Magn. Reson. Med. 30, 161–173 (1993).
Boynton, G.M., Engel, S.A., Glover, G.H. & Heeger, D.J. Linear systems analysis of functional magnetic resonance imaging in human V1. J. Neurosci. 16, 4207–4221 (1996).
Forman, S.D. et al. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn. Reson. Med. 33, 636–647 (1995).
Acknowledgements
We thank A. Bartels, A. Ghazanfar, K. Hoffman, A. Ishai, C. Kayser, G. Rainer, S. Smirnakis, L. Sugrue and K. Uludag for comments on a previous version of the manuscript; D. Blaurock for English editing; and S. Weber for fine-mechanic work. This study was supported by a long-term fellowship from the European Molecular Biology Organization awarded to A.S., and by the Max Planck Society.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
Neuronal response to stimulation of part of the visual field. (PDF 44 kb)
Supplementary Fig. 2
BOLD and neuronal response to stimulation of part of the visual field from 2 experiments with low-amplitude NBR and low-amplitude increases in neuronal activity. (PDF 126 kb)
Supplementary Fig. 3
The negative BOLD response is correlated with decreases in neuronal activity. (PDF 139 kb)
Supplementary Fig. 4
Region of interest adjacent to the NBR in V1. (PDF 22 kb)
Supplementary Fig. 5
Ratio of BOLD response to neuronal response. (PDF 45 kb)
Rights and permissions
About this article
Cite this article
Shmuel, A., Augath, M., Oeltermann, A. et al. Negative functional MRI response correlates with decreases in neuronal activity in monkey visual area V1. Nat Neurosci 9, 569–577 (2006). https://doi.org/10.1038/nn1675
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn1675
This article is cited by
-
Rat superior colliculus encodes the transition between static and dynamic vision modes
Nature Communications (2024)
-
Modeling the relationship between neuronal activity and the BOLD signal: contributions from astrocyte calcium dynamics
Scientific Reports (2023)
-
Tracing development of song memory with fMRI in zebra finches after a second tutoring experience
Communications Biology (2023)
-
Layer-specific, retinotopically-diffuse modulation in human visual cortex in response to viewing emotionally expressive faces
Nature Communications (2022)
-
Multilayer CVD graphene electrodes using a transfer-free process for the next generation of optically transparent and MRI-compatible neural interfaces
Microsystems & Nanoengineering (2022)